Figure 4.
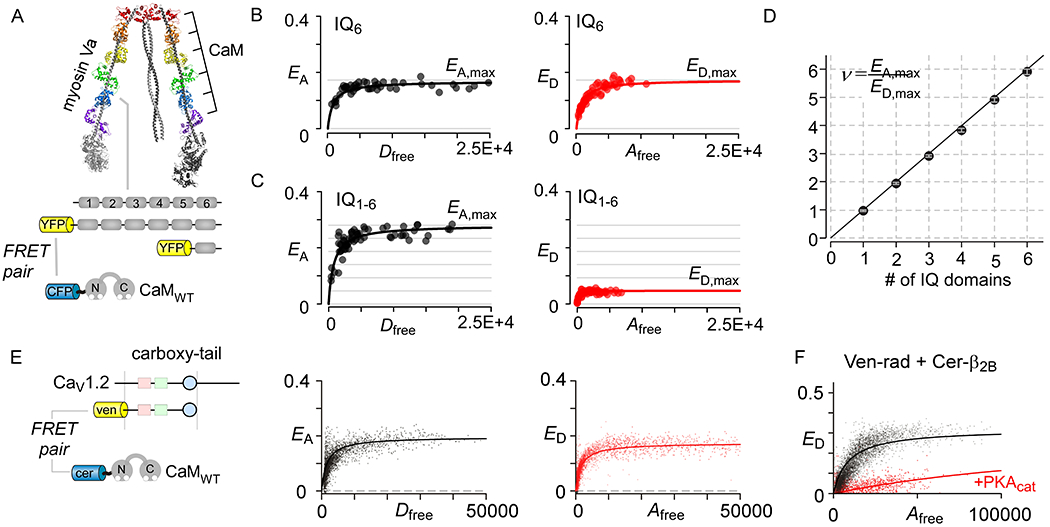
Application of FRET 2-hybrid analysis to deduce stoichiometry and binding. (A) Cryo-electron microscopy of Myosin Va shows the interaction of 6 CaM molecules with the neck domain that contains 6 tandem IQ domains (PDB code, 2DFS) (J. Liu, Taylor, Krementsova, Trybus, & Taylor, 2006). (B) FRET 2-hybrid analysis of a single IQ domain shows identical saturating values for acceptor-centric (EA,max) and donor-centric (ED,max) FRET efficiencies suggesting 1:1 binding. (C) Analysis of the entire neck region containing IQ1–6 shows that saturating value for acceptor-centric (EA,max) is ~ 6-fold higher than the donor-centric (ED,max) FRET efficiencies. This suggest that there are 6 donor molecules (i.e. CaM) per acceptor in the complex. (D) Correlation of estimated stoichiometries for various truncations of myosin Va neck region using FRET with the expected stoichiometry given the number of IQ domains in each truncation. (E) Flow-cytometric FRET 2-hybrid assay confirms robust interaction of Cer-tagged CaM with ven-tagged carboxy-tail segment of CaV1.2. As EA,max = ED,max, the stoichiometry is 1:1. (F) Flow-cytometric FRET 2-hybrid assay shows PKA-dependent change in binding of the Cerulean tagged CaVβ2B subunit with Venus tagged Rad. These findings illustrate the ability of FRET to resolve changes in binding due to signaling events. Panels B-D were reproduced with permission from Ben-Johny et al, 2016.
