Abstract
Özcan et al. (2021) and van Beljouw et al. (2021) characterize a novel Type III-E CRISPR-Cas subtype, composed of a single polypeptide with crRNA processing and sequence-specific RNA cleavage activities, that provides a new RNA knockdown tool for mammalian cells with fewer off-target effects than current technologies.
In their native context, CRISPR-Cas systems provide adaptive immunity for their prokaryotic host cells. They do this by acquiring short DNA sequences from invading threats, like viruses, and storing them as “spacers” in host genomic CRISPR arrays. The arrays are transcribed and processed into mature CRISPR RNAs (crRNAs), which form effector complexes with Cas proteins and then guide the effector complexes to cleave target DNAs or RNAs. Through use of engineered crRNAs, the highly specific nature of this nucleic acid cleavage has been harnessed for programmable DNA-targeting technologies—a monumental advance for which the 2020 Nobel Prize in Chemistry was awarded.
CRISPR-Cas systems are categorized into one of two classes based on the organization of their effector ribonucleoproteins—Class 1 systems are those where multiple protein subunits combine to form a single effector complex, whereas Class 2 systems are composed of a single effector protein. While the genetic simplicity of Class 2 systems is preferred for biotechnological applications, they make up just ~20% of CRISPR-Cas systems (Makarova et al., 2020). Additionally, while DNA-targeting systems have been developed into a suite of powerful genetic modification tools (Hsu et al., 2014), RNA-targeting CRISPR-Cas systems have only recently emerged as programmable tools (Terns, 2018). Class 1 Type III systems comprise the majority of natural RNA-targeting CRISPR systems, but it is the rarer Class 2 Type VI effectors, composed of the Cas13 protein and a crRNA, that have taken the lead as newly developed RNA knockdown and editing tools (Abudayyeh et al., 2017; Cox et al., 2017). However, cleavage by crRNA-Cas13 complexes is not limited to the crRNA-bound RNA target (Abudayyeh et al., 2016), and significant toxicity and off-target effects from Cas13-based systems have been noted in many cell types (Meeske et al., 2019; Wang et al., 2019). Thus, there is much interest in identifying new and better single-effector CRISPR-Cas systems capable of RNA targeting.
First described in 2019 (Makarova et al., 2020), the Type III-E systems appeared to be a promising alternative. Now, two studies (Özcan et al., 2021; van Beljouw et al., 2021) have characterized Type III-E CRISPR-Cas systems, demonstrating their applicability as highly-specific, programmable, all-in-one RNA-targeting systems (Figure 1). Expanding upon the previous identification of III-E systems,Özcan et al. searched 11.6 billion protein sequences from both collated and metagenomic sequence datasets, identifying 17 total Type III-E systems. While all other Type III systems are Class 1 multi-subunit effector complexes, Type III-E systems appear to be a fusion of canonical Type III Cas7 and Cas11 subunits into a single polypeptide. The resulting polypeptide is referred to as Cas7-11 by Özcan et al., and gRAMP (for giant repeat-associated mysterious protein, as coined by [Makarova et al., 2020]) by van Beljouw et al. This fusion of four Cas7 subunits (one with a large insert) and one Cas11 subunit results in the largest CRISPR-Cas single-effector found to date (1,300–1,900 amino acids). The genes encoding these Cas7-11 effectors are usually linked to a CRISPR array (found in 12/17 systems identified), and often (8/17) with a Cas1 adaptation protein—the majority of which (6/8) are fused to a reverse transcriptase domain, suggesting spacer acquisition occurs from RNA substrates (Figure 1). Consistent with this, van Beljouw et al. identified targets for 20 spacers in Type III-E arrays, revealing a strong bias for coding strands over template strands—a requirement for true RNA targeting activity. Intriguingly, the canonical counterpart of adaptation machinery, Cas2, appears absent in all but one Type III-E system, begging the question of how spacer uptake proceeds in these enigmatic systems.
Figure 1. Type III-E CRISPR-Cas systems are all-in-one RNA targeting tools.
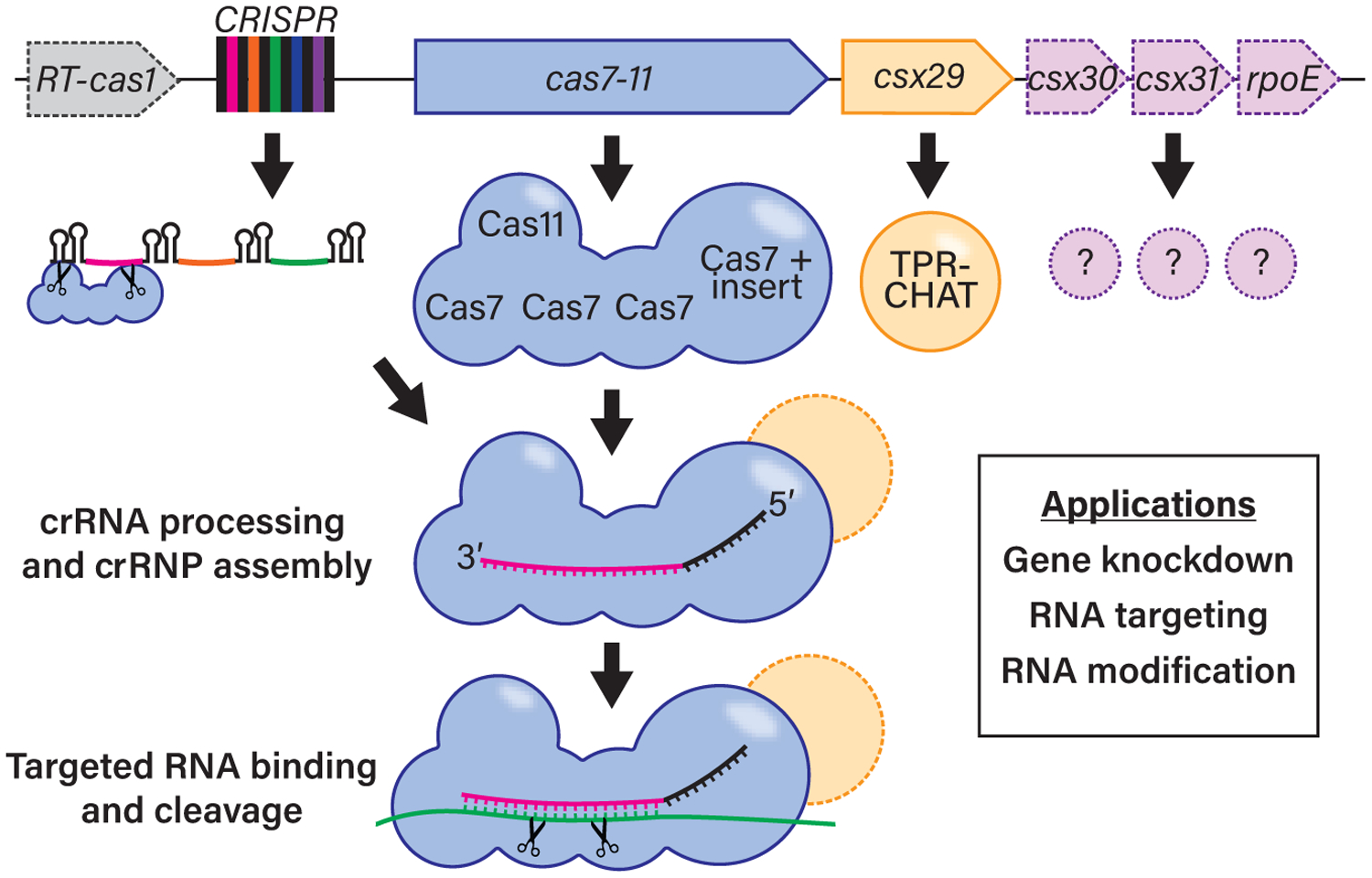
Two new studies (Özcan et al., 2021 and van Beljouw et al., 2021) reveal the rare Type III-E CRISPR-Cas systems are active in RNA targeting. The single effector protein (Cas7-11) is a fusion of four Cas7 domains (one with a large insert) with one Cas11 domain. This Cas7-11 protein cleaves CRISPR array transcripts to generate mature crRNAs and uses these as guides to cleave RNAs at two sites in a sequence-specific manner. A mysterious accessory protein, TPR-CHAT, with predicted caspase-like peptidase activity forms a complex with Cas7-11, but its function remains unknown (as do the functions of other less-commonly-observed accessory genes shown in pink). Özcan et al. (2021) develop Cas7-11 as a programmable RNA knockdown/editing tool that functions in mammalian cells with advantages over current RNA technologies.
For the vast majority of Type III systems, CRISPR array transcripts are processed into mature crRNAs by an accessory protein, Cas6. Here, both studies show that Cas7-11 processes crRNA transcripts to mature crRNAs without the aid of accessory proteins (Figure 1), reminiscent of the Type V Cas12 and Type VI Cas13 effectors. This crRNA processing results in a mature crRNA comprised of a ~20-nt guide element for target RNA recognition and a 5′ CRISPR repeat segment that is of variable length (from 14–28 nt, depending on the system investigated). Interestingly, the final 14 nt of the direct repeat RNA appear highly conserved among Type III-E arrays, suggesting that a 14-nt crRNA 5′ tag element may be a recognition motif for Cas7-11 protein interaction. Özcan et al. go a step further and recognize the cleavage site as a bulge in a predicted hairpin formed by the direct repeat RNA. This crRNA processing ability of the Cas7-11 protein appears independent from target RNA cleavage, being metal independent and proceeding in the presence of mutations, which abolish target RNA cleavage. In contrast to crRNA processing, cleavage of target RNAs is metal-dependent, highly sequence-specific, and occurs at two sites 6 nt apart directed by Cas7 domains. This 6-nt spacing is reminiscent of cleavage by canonical Type III systems, which results from the physical spacing of adjacent Cas7 subunits. This suggests that Cas7-11 may have retained ancestral structural features despite novel gene organization. The two cleavage sites directed by Cas7-11 do not appear to be functionally equivalent, with one site requiring a particular conserved Cas7 aspartate residue. How the second site is generated is unclear, though it is likely still catalyzed by a separate Cas7 domain. The role of the two other Cas7 domains remains unknown. Perhaps these have taken on a crRNA processing role or are now mainly structural in function. The function of the large insert in one Cas7 is also mysterious.
Type III systems are often accompanied by accessory proteins involved in downstream activities (e.g., the non-specific nucleases Csm6/Csx1, activated by cyclic oligoadenylate [cOA] signaling molecules produced by the Type III effector complex upon target RNA binding). cOA production has been lost in Cas7-11, but a peculiar accessory protein appears to have been adopted (Figure 1). Csx29 is found in 15/17 identified Type III-E systems and encodes a tetratricopeptide repeat domain (TPR) fused to a caspase-like protease (CHAT), suggesting a potential for caspase-like induction of cell death and/or protease activities. TPR-CHAT forms a stable complex with Cas7-11 (van Beljouw et al., 2021); however, neither study was able to show any ancillary function. Rather, the protein inhibited Cas7-11 RNA targeting in vitro and MS2 phage interference in an E. coli system (Özcan et al., 2021) or had no apparent effect on target RNA cleavage in vitro (van Beljouw et al., 2021). The functions of other accessory proteins, Csx30 (found in 10/17 systems), Csx31 (8/17), and RpoE (8/17), are also unknown as yet, though RpoE shows sequence similarity to the sigma24 transcription factor.
Despite the conservation of these accessory proteins, they are not required for RNA targeting of Cas7-11. Özcan et al. were able to use Cas7-11 alone to target mRNAs in the heterologous contexts of E. coli and mammalian cells. Here, sequence-specific targeting resulted in RNA knockdown and decreased protein expression. Additionally, the specificity of the Cas7-11 system resulted in significantly fewer off-target effects in mammalian cells than the classical RNA interference using short hairpin RNAs, or the more recent Cas13 technologies, indicating that Cas7-11 may be a superior RNA knockdown method. Moreover, fusion of Cas7-11 to an adenosine deaminase (ADAR2) resulted in site-specific A-to-I modification of RNAs in vivo, albeit at low frequency (2%–8% of transcripts). This is somewhat reflective of the variable knockdown efficiencies observed with mRNA targeting (25%–80%, depending on cell type, guide RNA, target RNA, etc.). While the tested Cas7-11 proteins seem to exhibit high specificity—indeed, higher specificity than other previous RNA targeting techniques—they do so with less than desirable target RNA cleavage efficiency. Hence, these systems provide an exciting starting point for further engineering of the protein or crRNA to increase RNA knockdown efficiency and to develop further methods for novel RNA targeting applications.
Many questions remain to be answered about these enigmatic variant CRISPR systems, including how crRNA processing proceeds, the functions of the four accessory proteins and large Cas7 insert, how the systems acquire new spacers, and whether any of the other identified III-E Cas7-11 orthologs will exhibit improved RNA-targeting properties. Despite these outstanding questions, Type III-E systems clearly present not only an exciting biotechnological innovation but also an intriguing evolutionary innovation. These studies highlight the importance of exploring novel sequences and understanding the functional properties of rare, derived CRISPR-Cas subtypes.
ACKNOWLEDGMENTS
M.P.T. acknowledges funding from the NIH (R35GM118160).
REFERENCES
- Abudayyeh OO, Gootenberg JS, Konermann S, Joung J, Slaymaker IM, Cox DB, Shmakov S, Makarova KS, Semenova E, Minakhin L, et al. (2016). C2c2 is a single-component programmable RNA-guided RNA-targeting CRISPR effector. Science 353, aaf5573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abudayyeh OO, Gootenberg JS, Essletzbichler P, Han S, Joung J, Belanto JJ, Verdine V, Cox DBT, Kellner MJ, Regev A, et al. (2017). RNA targeting with CRISPR-Cas13. Nature 550, 280–284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cox DBT, Gootenberg JS, Abudayyeh OO, Franklin B, Kellner MJ, Joung J, and Zhang F (2017). RNA editing with CRISPR-Cas13. Science 358, 1019–1027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsu PD, Lander ES, and Zhang F (2014). Development and applications of CRISPR-Cas9 for genome engineering. Cell 157, 1262–1278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Makarova KS, Wolf YI, Iranzo J, Shmakov SA, Alkhnbashi OS, Brouns SJJ, Charpentier E, Cheng D, Haft DH, Horvath P, et al. (2020). Evolutionary classification of CRISPR-Cas systems: a burst of class 2 and derived variants. Nat. Rev. Microbiol 18, 67–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meeske AJ, Nakandakari-Higa S, and Marraffini LA (2019). Cas13-induced cellular dormancy prevents the rise of CRISPR-resistant bacteriophage. Nature 570, 241–245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Özcan A, Krajeski R, Ioannidi E, Lee B, Gardner A, Makarova KS, Koonin EV, Abudayyeh OO, and Gootenberg JS (2021). Programmable RNA targeting with the single-protein CRISPR effector Cas7-11. Nature 597, 720–725. [DOI] [PubMed] [Google Scholar]
- Terns MP (2018). CRISPR-Based Technologies: Impact of RNA-Targeting Systems. Mol. Cell 72, 404–412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Beljouw SPB, Haagsma AC, Rodríguez-Molina A, van den Berg DF, Vink JNA, and Brouns SJJ (2021). The gRAMP CRISPR-Cas effector is an RNA endonuclease complexed with a caspase-like peptidase. Science 373, 1349–1353. [DOI] [PubMed] [Google Scholar]
- Wang Q, Liu X, Zhou J, Yang C, Wang G, Tan Y, Wu Y, Zhang S, Yi K, and Kang C (2019). The CRISPR-Cas13a Gene-Editing System Induces Collateral Cleavage of RNA in Glioma Cells. Adv. Sci. (Weinh.) 6, 1901299. [DOI] [PMC free article] [PubMed] [Google Scholar]


