ABSTRACT
Developing and implementing new immunization policies in response to shifting epidemiology is a critical public health component. We adopted a mixed-methods approach (via narrative literature review [101 articles] and 9 semi-structured interviews) to evaluate policy development in response to shifting measles epidemiology in six European countries (Italy, Belgium, Germany, Romania, UK, and Ukraine); where policies and strategies have evolved in response to country-specific disease and vaccination patterns. Periodic outbreaks have occurred in all countries against a background of declining measles-containing-vaccine (MCV) uptake and increasing public vaccine hesitancy (with substantial regional or social differences in measles burden and vaccine uptake). Health-care worker (HCW) vaccine skepticism is also seen. While many outbreaks arise or involve specific susceptible populations (e.g., minority/migrant communities), the broader pattern is spread to the wider (and generally older) population; often among incompletely/non-vaccinated individuals as a legacy of previous low uptake. Immunization policy and strategic responses are influenced by political and social factors, where public mistrust contributes to vaccine hesitancy. A strong centralized immunization framework (allied with effective regional implementation and coherent political commitment) can effectively increase uptake. Mandatory vaccination has increased childhood MCV uptake in Italy, and similar benefits could be anticipated for other countries considering vaccine mandates. Although possible elsewhere, socio-political considerations render mandating impractical in other countries, where targeted immunization activities to bolster routine uptake are more important. Addressing HCW skepticism, knowledge gaps, improving access and increasing public/community engagement and education to address vaccine hesitancy/mistrust (especially in communities with specific unmet needs) is critical.
KEYWORDS: Measles, routine immunization, decision-making, policy
Introduction
Developing and implementing new immunization policies in response to shifting epidemiology is a critical component of public health. The use of measles-containing vaccines (MCVs) in routine national immunization programs (NIPs) has led to substantial progress in global control and measles elimination in high- and low-burden regions.1,2 Across Europe, where MCVs have been available for over 50 years, success has been achieved through programmatic targets to increase the uptake of the first dose of measles-containing vaccine (MCV1) in infants, and second-dose (MCV2) in older children.3,4 Supplemental immunization activities (SIAs) targeting susceptible groups and individuals who have missed out on scheduled recommended MCV immunizations are an additional crucial strategy.3,4 High vaccination coverage (VC) of >95% for both doses is considered necessary to achieve the population (herd) immunity threshold required to achieve and maintain measles elimination; by 2017, 43 (91%) countries/states in Europe had interrupted endemic measles virus transmission for ≥12 months, with 37 (70%) having verified elimination (defined as sustained interruption of endemic measles virus transmission or ≥36 months).3,5
Nevertheless, progress in measles control and elimination has stalled, with a substantial resurgence in Europe (and indeed globally) in recent years (with more than a tenfold increase in cases between 2016 and 2018),3 and it is likely that the current COVID-19 pandemic may lead to further resurgence. While the dramatic increase was particularly driven by a substantial outbreak in Ukraine, the broader trends reflect uneven progress across Europe, with numerous measles outbreaks observed, with most cases occurring in unvaccinated or incompletely vaccinated individuals.3,5,6 MCV uptake in many European countries has declined over the past decade,7 and while influenced by many factors including vaccine availability and access, this decline has been accompanied by increasing vaccine hesitancy associated with skepticism and reduced public confidence in vaccines and vaccination policies.8,9
A range of policies exist to increase measles vaccine uptake, including strengthening of existing NIP programs at a regional or national level, with SIAs and more tailored immunization programs targeting specific susceptible communities. In addition, mandatory vaccination policies are in effect in a number of European countries, including France, Italy and Germany, where there is a legal requirement to ensure uptake of recommended pediatric vaccines (including MCV).10–12
This study is a narrative analysis of evolving measles epidemiology and immunization strategies and policies in six European countries (Italy, Belgium, Germany, Romania, UK, and Ukraine), each of which presents different approaches and challenges in measles control. Key drivers and barriers that govern policy and implementation are also identified.
Methods
A ‘mixed-methods’ approach was adopted, with a literature review of research across six countries (Italy, Belgium, Germany, Romania, UK, and Ukraine); country selection was broadly based on the desire to consider different immunization system archetypes, e.g., centralized or decentralized immunization system or recognized differences in mandatory immunization policy, and countries considered in transition, such as Ukraine. Romania was included as a country with a substantial measles resurgence in recent years. This was complemented by semi-structured interviews with key informants on measles immunization policy from two of these countries (Italy and UK); each representing different approaches toward immunization policy and delivery.
Literature search
PubMed and Web of Science databases were searched to identify literature related to measles epidemiology and disease burden, the evolution and adaptation of immunization programs, and potential barriers in the selected countries, using the following search terms: (immunization OR vaccine hesitancy OR vaccine confidence OR vaccine-preventable disease OR outbreak response) AND (measles) AND (Germany OR Italy OR Belgium OR Romania OR United Kingdom OR Ukraine). Although comprehensive, the literature search was not systematic. The purpose was to identify relevant publications to help form a coherent overview of measles burden and immunization policies, rather than to perform a formal critical analysis of the published literature.
Results were limited to articles published in English between 2000 and 2020; a period when most European countries have collected robust data.
Interviews
Semi-structured interviews with experts involved in measles immunization programs were conducted to provide contextual insights. Due to resource constraints, interviews were limited to two countries (Italy and UK) each representing a distinct model of public health system. A purposeful sampling approach was adopted, selecting four to five key informants from each country,13 with informants identified on the basis of their recognized involvement in measles immunization programs (ascertained through their publication record and/or involvement in NIPs) complemented with subsequent referrals using snowball sampling. Prior to the interviews, informants completed a structured questionnaire (developed and pre-tested before use) to gather their perceptions and opinions on their countries immunization framework and policies to inform subsequent discussion (Supplementary information). All interviews were conducted in English by one researcher (IV) via online video calls or telephone, recorded and transcribed, and evaluated through thematic content analysis,14 whereby themes were identified and coded, with recurrent issues raised by multiple informants generating key thematic categories.
Analysis
Findings from the literature analysis were described from a narrative perspective to provide a holistic view of the broad range of topics and issues described in published data, then summarized in a matrix format to highlight key themes within and across countries as a framework analysis. Three broad themes were chosen reflecting the policy triangle of: 1) content (the nature of the problem that the specific immunization policy is meant to address), 2) context, and 3) process (organizational mechanisms/actions required to implement the policy).15
Ethical considerations
The qualitative aspect of this study was approved by the MSc Research Ethics Committee of the London School of Hygiene & Tropical Medicine (LSHTM) and in compliance with local ethical requirements within informant countries (Ethics reference number 21322). All interviewed participants provided prior informed consent prior to interview and allied data collection.
Results
The literature search identified 101 articles reviewed and included in qualitative synthesis and analysis (Figure 1). A synthesis of key observations drawn from the literature review is presented below. An overview of measles burden and shifting epidemiology (much of which is driven by disease outbreaks) and vaccine uptake,16–21 in all six countries is shown in Figure 2. The evolution of measles vaccine used across the six countries and the current measles vaccination schedules 21–23 are shown in Figure 3. A narrative of key aspects of measles immunization policy and disease burden for each country is as follows.
Figure 1.
Flow diagram for study selection.
Figure 2.
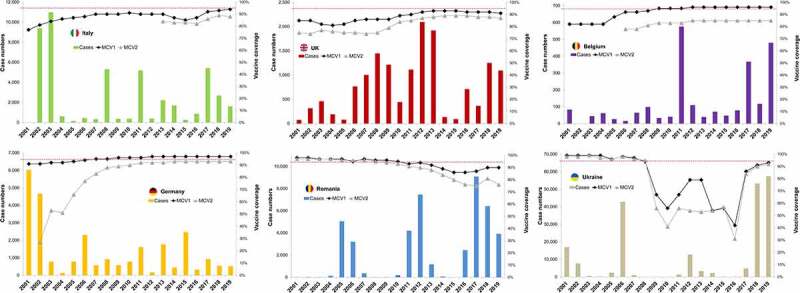
Measles case numbers and vaccine coverage for first and second MCV dose in selected countries 2001–2019.
Case numbers as reported by the WHO for 2001–2019, supplemented by individual WHO-EU measles and rubella elimination country profiles.16–21 Vaccine coverage represents official country estimates for 2001–2019 as reported by the WHO.16 The red line indicates 95% coverage in the age-eligible target populations.
Figure 3.
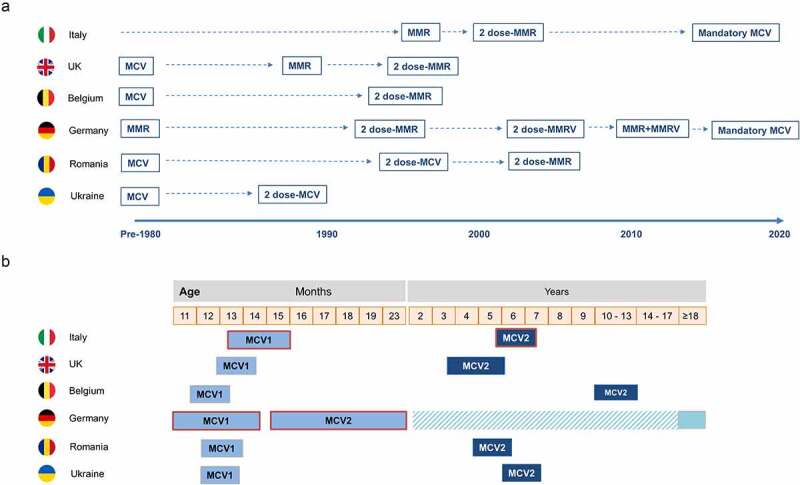
Current measles vaccination schedule in selected countries.
Scheduled vaccinations as reported by ECDC and other sources.21–23Cross-hatch indicates mandatory catch-up recommendation for Germany. MCV, measles-containing vaccine; MCV1, first dose of measles-containing vaccine; MCV2, second dose of measles-containing vaccine; MMR, measles-mumps-rubella-vaccine; MMRV, measles-mumps-rubella-varicella vaccine.
Italy
Healthcare is decentralized; immunization policy, programs, and targets are set centrally (at a national level) with responsibility for implementation and delivery at the regional level.24 While some childhood vaccines have been mandatory for many years (diphtheria, tetanus, hepatitis B and polio) others, including measles, have – until recently – only been recommended but not compulsory. Measles vaccination (using MMR) was introduced in the NIP as a recommended childhood vaccine in 1999. A 2-dose strategy was implemented in 2003, with MCV1 given at 12–15 months of age and MCV2 at 5–6 years.25 The target of achieving 95% VC for each MCV dose has yet to be achieved. In 2010, VC for MCV1 was 91% (and lower for MCV2, although not routinely monitored at that time), after which a gradual decline was seen (with MCV1 falling to 85–87%, and MCV2 uptake to 83% (2014–16)).26,27 Substantial regional variations were evident, with uptake greater in the North and lowest in the South.25,28
While measles cases fell dramatically from 2004 onwards, numerous outbreaks involving susceptible populations have occurred, leading to periodic and often marked increases, notably in 2008, 2011, and 2017.29–31 While clusters involving susceptible communities with low vaccine uptake (chiefly Roma/Sinti communities) are well known 32,33 and widely commented upon in the media,34–36 most outbreaks have involved unvaccinated Italian nationals, in particular infants <1 year and unvaccinated older children and adults;29–31 including health-care workers (HCWs) who act an important vector in nosocomial infection.37,38 Most recently, a significant increase occurred in 2017 (5,400 cases), driven by outbreaks across most of the country, although the greatest burden was in central regions (especially Lazio) and the North.31 In contrast to previous outbreaks, Roma/Sinti populations were not a focus of the 2017 outbreaks; the majority of those affected were unvaccinated Italian nationals (88%), and 75% were ≥15 years of age.31
In June/July 2017, the government legislated for an additional six vaccines to become mandatory (against measles, pertussis, Haemophilus influenzae type b [Hib], mumps, rubella and varicella). Non-compliance would result in exclusion of unvaccinated children from nurseries and schools and financial penalties to parents or guardians.27 Although the legislation was prompted by the significant measles outbreak, it also aimed to increase the uptake of recommended vaccines, including MCVs, which consistently lagged behind the pre-existing mandatory vaccines (where VCs were approximately 95%).27,39 This action polarized public opinion, with considerable political debate (and anti-vaccination rhetoric) in the subsequent 2018 election,40 although the majority of Italians approved the new regulation.41 This policy, which also allocated greater resources for immunization activities within local public health services,42 had an immediate impact on measles immunization rates, with VC for MCV1 and MCV2 each >5% higher in 2018 compared to 2016,28 with high MCV1 uptake in previously unvaccinated children born in 2011–15,28,43 and 94% MCV1 uptake in 2019.44 The decline in case numbers in 2018 and further still in 2019 suggests an impact of these measures (Figure 2).
While the mandatory vaccination legislation should ensure VC among children and adolescents, including those who missed out on previously recommended vaccination, additional immunization activities are needed to increase vaccination coverage rates in other populations (expectant mothers, Roma/Sinti communities, HCWs) to maintain high population/herd immunity.45–50 Stronger regional surveillance can help target local immunization needs (and barriers) accompanied with SIAs, and stronger communication strategies. These additional strategies are a primary focus of the new Italian Measles and Rubella Elimination Plan 2019–2023.25,28
UK
UK immunization policy is centralized, directed through the Department of Health, with implementation then delivered under the autonomy of health authorities in each of the four UK countries (England, Scotland, Wales, and Northern Ireland).24 Specific policies are guided by recommendations from an independent expert advisory committee, the Joint Committee on Vaccination and Immunization (JCVI). Public engagement is limited but public reaction can occasionally contribute to decision-making, e.g., when the public responded negatively to initial decisions not to recommend routine infant meningitis B vaccination, which allied with a broader health economic assessment led to subsequent recommendations for NIP inclusion.51
Childhood measles vaccination was first introduced in 1968, with MMR used since 1988 initially as a single-dose in infants, with high uptake (90% by the early 1990ʹs).52,53 A 2-dose schedule was introduced in 1996 targeting (infant and school-age children).23,54 Vaccine uptake in the late 1990ʹs was significantly hampered by widespread media reporting and misrepresentation of data alleging linkage of MMR vaccine with autism, which reduced public confidence in vaccine safety and MCV1 uptake in England declined from 92% in 1995 to 81% in 2003.52 Nevertheless, measles incidence remained low (with most cases being imported) and was considered eliminated until 2005, after which, an increase in case numbers in 2006 led to re-establishment of measles transmission in 2006.52,53 Although MCV uptake showed some recovery, measles outbreaks occurred between 2007 and 2013 (Figure 2), in part explained by a marked age-shift in cases as a consequence of lower uptake in previous years.52,55–61
In this period, notable outbreaks were reported in specific UK regions; for example, those in North West England (notably Liverpool) (in 2008–2009 and 2012–2013),55–58 and in Wales (in 2012/2013),52 while others involved susceptible communities such as the orthodox-Jewish, Irish travelling, and Roma communities.59–61 A subsequent decline in cases in 2014/15 led to re-instatement of WHO measles elimination status in 2016; however later resurgence, including outbreaks during 2017/18 involving Romanian and Roma communities,62 again led to re-establishment of endemic measles transmission in 2019 and England lost their measles elimination status. A feature common to most outbreaks was that while many cases involved infants <1 year who were too young to be vaccinated, a substantial number involved older children who, while age-eligible, were also either unvaccinated or had received only MCV1; most adult cases were either unvaccinated or of unknown vaccination status.55,56,58,63
Targeted SIAs and immunization campaigns to address missed vaccinations in these and other similar outbreaks have generally been effective in limiting outbreak spread or in increasing subsequent uptake in under-vaccinated communities.55,56,58,63,64 As data from Liverpool indicate that socioeconomic inequalities are evident in areas with lower or delayed vaccine uptake and timeliness, these populations could also benefit from specific campaigns.65 Understanding barriers and specific community concerns in susceptible populations can help to inform appropriate interventions. For example, in the UK Roma community, language difficulties and healthcare access may be more relevant than safety concerns,62 while in other groups, e.g. orthodox Jewish communities, safety may be more important.66
Although MCV uptake eventually recovered following the initial MMR-autism controversy, vaccine hesitancy and mistrust persisted in some settings as reported around the Liverpool outbreaks,67 although one study suggests that more recent declines are less strongly associated with the autism controversy.68 Public confidence remains an important consideration, and no doubt a factor in recent renewed discussion of mandatory vaccination in the UK (driven both by recent resurgence along with similar policy changes elsewhere in Europe),69 although there seems to be little appetite among UK policy-makers for mandates, in light of potential public resistance and enforcement impracticalities.69,70
Belgium
Three different communities co-exist across Belgium’s four administrative regions; the Flemish (approximately 60% of the population, predominantly in Flanders, northern Belgium) and the French (predominantly in Wallonia, southern Belgium) with both present in the bilingual Brussels-Capital Region (which also has a substantial international community); and a small German community in the eastern part of the country. While the NIP is co-ordinated at a national level (under the aegis of the Federal Public Health Service through the NITAG) where recommendations are drafted, Belgium’s vaccination programs are organized at the subnational level. Indeed, disease prevention in health care, and hence immunization policy, is a duty and responsibility of the Communities and the Regions. Polio immunization is mandatory for all, while in the French community vaccination against diphtheria, whooping cough, Hib, measles, rubella and mumps are recommended, and only mandatory for younger children attending creches or public childcare centers.24 In contrast, although recommended, these vaccines are not compulsory within the Flemish community for day care access.
Childhood measles vaccination was introduced in 1985, and a two-dose MMR strategy in 1994; MCV1 is given at 12 months of age and MCV2 at 10–13 years. Periodic catch-up campaigns targeting children missing out on scheduled doses and adults aged 20–45 years with uncertain immunization status are also implemented (during the annual WHO EURO vaccination week).24 Vaccine uptake is periodically assessed (in 2005, 2008, 2012 and 2016), with MCV1 uptake maintained around 95% since 2009 (nationally and in all four administrative regions). MCV2 coverage is lower (85%), where uptake shows distinct regional/community variation; higher in Flanders (93% in 2012 and 2016) and far lower in Wallonia and Brussels (75% in 2012 and 2016).71
Measles cases remained relatively low between 2001 and 2010, although a localized outbreak occurred in Antwerp, Flanders in 2007–2008; 137 cases, mainly within orthodox Jewish communities (with possible linkage to allied communities in the UK and Israel).72,73 In 2011, a total of 576 measles cases were reported (a recent historical high), involving several distinct outbreaks, including one in Ghent, Flanders, with a total of 65 confirmed cases principally involving children attending anthroposophic schools (that follow the humanistic teachings of Rudolf Steiner), where MCV uptake in pupils was 45–50%.74 Efforts to limit spread included early case isolation, a school vacation and a limited vaccination of susceptible children.74 Thereafter from 2012 to 2016 case numbers declined, and then a substantial outbreak involving 289 cases developed in Wallonia from late-December 2016 through May 2017.75,76 Imported cases (from Romania) were linked to 51 cases.75,76 The majority of cases were either unvaccinated (40%) or with unknown vaccination status (46%). HCWs accounted for 12% of cases; 17% were unvaccinated and 47% were of unknown status.75,76 Although case numbers were low in 2018 (117 cases), resurgence was apparent in 2019, with 480 cases reported (Figure 2).
Surveys have shown that attendance at a maternal and child health clinic is a major influence on vaccine uptake in Brussels and Wallonia, where free vaccine access also reduces socioeconomic inequality, and vaccine refusal is rare.77 There are limited recent data on public perception of vaccines in Belgium (including any differences in French or Flemish communities). However, differences in uptake within these communities, and the existence of groups with recognized lower coverage would suggest that perception and vaccine hesitancy/refusal remain a factor in apparent disparities in uptake. A recent study evaluating vaccine hesitancy in French-speaking GPs reported moderate-to-high hesitancy in 50% of those surveyed, and hesitancy impacted measles vaccine recommendations; only 72.4% of physicians with moderate-to-high hesitancy recommended MMR vaccination for unimmunized adolescents or young adults compared to 92.7% of the physicians with little or no hesitancy.78
Germany
Measles vaccination is long-established, although policy differences existed prior to 1990 re-unification; the German Democratic Republic (formerly Eastern Germany) had a mandatory vaccination policy since 1970 while in the Federal Republic of Germany (formerly Western Germany) vaccines were only recommended, with monovalent vaccine introduced in 1974, and MMR in 1980. Two-dose schedules have been used across re-unified German since 1991.79,80 Immunization policy is decentralized; based on recommendations from the German Standing Committee on Vaccination at the Robert Koch Institute (STIKO), with implementation devolved to the 16 individual federal states.80,81 Since 2001, MCV1 has been recommended at 11–14 months and MCV2 at 15–23 months of age; MMRV was introduced in 2006 (although due to a potential increased risk of febrile seizure, since 2011 MMRV is used only for the second MCV dose).82 In 2010, free-of-charge catch-up vaccination of all adults (born after 1970) was also recommended, if vaccination status is unclear, or when individuals had never received any or had only one dose of MCV.80 Mandatory measles vaccination was recently introduced (in 2020) in response to under-vaccination and serious measles outbreaks.
Historically, MCV uptake was higher in Eastern Germany than in the West, and following reunification, uptake remained higher in Eastern states into the 2000's.81,83 MCV1 uptake has been consistently high in the East since 2000, although second-dose uptake lagged considerably.80,81,83–85 There are poorer physician and public attitudes toward vaccination and lower uptake in the South and in the Northwest regions, which have also seen the worst measles outbreaks.80,81,83,85 As elsewhere, physician recommendation is a major determinant of infant vaccine uptake in Germany, with attendance at a childcare unit also being a strong positive influence. Higher levels of parental education may negatively affect uptake in some regions, e.g., Würzburg.86
A substantial measles outbreak in 2006 in the Northwest (>1700 cases) chiefly affected unvaccinated older children.87–89 As seen elsewhere, communities with low vaccine uptake (anthroposophical communities, Roma and other migrants) have been the focus of a number of outbreaks, often with subsequent transmission to the broader population. Notable anthroposophical-associated outbreaks occurred in Bavaria in 2007,90,91 and also in 2008 (the latter imported from a related community in Salzburg, Austria, where a broader outbreak was taking place),92 with subsequent spread across southern Bavaria.93 Further anthroposophical school outbreaks occurred in 2010 in Berlin,94 and in Essen,95,96 and again in Berlin in 2011, although there the initial index case was a pupil from a conventional school.97 Outbreaks involving Roma and other migrant communities have had substantial impact both within and beyond Germany.98–101 In late 2008, an adult Roma contracted measles visiting London, and on return was a source of widespread outbreaks in Hamburg and across Saxony, via nosocomial transmission to patients attending emergency care services and also to the Roma community. The identified measles strain (D4-Hamburg Strain) was then associated with widespread onward transmission via Roma travelers across Europe from 2009 to 2011; including the Bulgarian epidemic (25,000 cases from April 2009–December 2011)102 and to later re-importation into Germany via Bulgarian Roma migrants in 2010.98,99 Migrant asylum seekers in Berlin were the likely initial source of the ‘D8-Rostov-Don’ strain imported into Berlin in 2014; while fellow asylum seekers were initially affected, spread into the resident Berlin population resulted in the most significant outbreak in Germany in 20 years with 1,344 cases from October 2014–August 2015.100,101
Mandatory immunization has been a matter for prolonged debate in Germany, with the initial prevailing opinion not to do so, relying more on the ethical and social responsibility of the general population.103,104 However, against this background of measles resurgence in 2015 (and the failure to achieve elimination status by this date) and recognized vaccination gaps in adults aged 18–40 years, in May 2019 the government proposed mandatory measles vaccination, which was approved by parliament in late 2019 and implemented in March 2020. Measles immunization is compulsory not only for children but for anyone born after 1970 (unless protected by previous measles exposure, especially staff in childcare and education and all HCWs. Non-compliance can result in exclusion from education/employment and financial penalties.26,105 Limited data indicate that a majority of surveyed physicians are in favor of this policy,105 although the early impact on vaccine uptake and measles burden remains to be evaluated.
Romania
The NIP includes recommendations for age-based child and adolescent vaccinations, and risk-based vaccinations in adults. At present, vaccines are recommended but non-mandatory (although some legislation for mandatory childhood vaccination was considered in 2017).24 All childhood vaccines are free of charge. NIP implementation is supervised at a district level, spanning 41 districts plus Bucharest).24
MCV1 was introduced in the NIP in 1979 and a 2-dose monovalent MCV1/MCV2 strategy in 1994, leading to substantial reductions in incidence rates,106,107 although periodic outbreaks occurred, with >33,000 cases in 1996–1998, mostly in unvaccinated infants and older children as a consequence of low MCV2 coverage.107 In response, Romania implemented enhanced surveillance and a nationwide immunization campaign, vaccinating 2.1 million 7–18 year-olds.107,108 MMR vaccination was introduced in 2004, with MCV1 given at 12–15 months and MCV2 initially given at 6–7 years of age (and at 5 years of age since 2015).106
While VC for both doses was consistently >95% from 2001 to 2009, this was followed by notable continued declines; in 2015 MCV1 and MCV2 uptake was 86% and 80%, respectively, with further decline in second-dose uptake (75% in 2017).16,106 MCV1 and MCV2 uptake in 2019 was 90% and 76%, respectively.16 These are national data, and substantial variations exist at an administrative district level.109 A 2015 assessment using the WHO measles risk assessment tool, considered 27 of these 42 districts (64%), mainly districts in western Romania and Bucharest in the southeast, being at very high/high outbreak risk, chiefly on the basis of low VC and suboptimal surveillance quality.110 Substantial variation across districts is also seen in vaccine uptake achieved with SIAs.106
The decline in measles VC across 2009–2017 was accompanied by two major epidemics, in 2011–2012 and then the prolonged 2016–2018 epidemic, the latter principally due to the B3 genotype, with epidemiological linkage indicating this genotype as a major cause of outbreaks in other European counties (including Belgium as described above).75,111 This most recent outbreak has involved almost 18,000 cases (with the greatest incidences in infants and children <5 years),109 and principally affected districts in the western part of the country, although notable outbreaks were also seen in some central and southern districts.106,112 A high number of fatalities (64) occurred, mainly in children <5 years.112 In both epidemics, the vast majority of cases (approximately 80% in 2011–2012 and 93% in 2016–2018) were in unvaccinated individuals (including a large proportion of young infants not yet eligible for MCV1 vaccination).106,109,112
Public health responses to these outbreaks have involved SIAs and catch-up campaigns, principally targeting children. In 2011, MMR vaccination was offered to all children aged between 7 months and 7 years, regardless of vaccination status (reaching over 4,500 children).106,111 In 2016, in response to the continued epidemic, additional SIAs targeting children ≤9 years were rolled out in outbreak areas, and then nationwide with mixed responses; overall only 31% of the eligible children were vaccinated.113 In general, uptake was greatest for MCV1 dosing in children <5 years, but with a far lower MCV2 uptake in 5–9-year-olds. Substantial regional variation in uptake was apparent, with uptake for either dose below 30% in some western districts with high measles case numbers.106 Although a 2017 survey indicated that the 83% of the public consider the national infant immunization strategy to be beneficial, broader concerns remain regarding public confidence in health-care policies and implementation106 and in vaccine safety.114 Vaccine refusal remains an important barrier to greater MCV uptake in eligible children, especially in urban areas, both for scheduled vaccinations and for those targeted by recent SIAs.109,110,113
Ukraine
Two-dose monovalent measles childhood vaccination was long-established in the former USSR, with MCV1 (at 12–15 months of age) introduced in 1969 and MCV2 (at 6 years) in 1986 with this schedule continued following USSR dissolution and separation of Ukraine in 1991.115,116 High reported vaccine uptake (>90%) was a feature before and after this transition, although the quality of these VC estimates has been questioned.117,118 While measles incidence remained low after separation, periodic outbreaks occurred (in 1993, 1996 and 2001) and then a far substantial outbreak in late 2005 through most of 2006.116,117 The origin of this latter outbreak focused in Kiev, but then spread across all 27 administrative territories,117 with over 44,000 cases reported (constituting 80% of all European cases in 2006).116 An important feature was the high proportion of cases in 15–29 year-olds, many of whom had received two vaccine doses.116,117 There was some evidence of lower than expected vaccine effectiveness, possibly due to various factors such as poor quality control during vaccine production or in cold-chain distribution, with an adverse impact upon population immunity thresholds,116 although inaccurate reporting of assumed coverage may also have played a part. Attempts to address vaccination gaps in 2008 via a national mass immunization campaign targeting 15–29 year-olds had an unfortunate event, with one vaccinee death, which – although assessed not to be caused by the vaccination – along with other adverse event reports, raised safety concerns amidst widespread adverse ‘anti-vaccination’ media reporting on safety and broader vaccine procurement matters.4,118 A poorly coordinated political response led to the cancellation of this campaign, and continued public mistrust of vaccine quality and safety had an adverse effect on all childhood vaccine uptake in subsequent years.115,118 Dramatic declines in national MCV1 and MCV2 uptake were seen in 2009, and remained low with a further marked decline to <50% in 2016.18,119,120 Declining VC was accompanied by a substantial outbreak in 2012, although as elsewhere, regional variations in uptake and measles incidence are seen.120 While great efforts to improve the uptake led to significantly higher VC in 2017 (for both doses) which continued in 2018/2019, this remains lower than the desired 95% threshold.18,119 That and the far larger susceptible unvaccinated or incompletely vaccinated population as a legacy of poor uptake in previous years led to an extensive sustained epidemic, beginning in 2017 (4,781 cases, focused mainly in the most western and southwestern regions) with dramatic escalation and eastward progression in 2018 and 2019 (53,219 and 57,282 cases respectively).17–21
This pattern has been heavily influenced by a complex array of systemic and social factors. The poor vaccine uptake from 2009 until relatively recently was chiefly driven initially by continued public skepticism around vaccine quality and safety, triggered by the 2008 events, and also continued vaccine supply issues, further compounded by the upheaval associated with political developments since 2014 (with Russia’s annexation of Crimea and continued conflict in the eastern Donbas region). These culminated in the dramatically poor uptake observed in 2016 (Figure 2) seen not only in measles vaccination; uptake of all routine childhood vaccines also markedly fell in 2015/16.18,119–121 Alongside this, continued dissatisfaction with health-care system and services, and the recognized corruption within society (and within healthcare) fosters continued distrust for authorities and institutions.122,123 Continued vaccine hesitancy in physicians and other HCWs has also been highlighted.124 While health-care services (including immunization) are free-of-charge, a gray health-care economy exists, and informal public out-of-pocket expenditure has been consistently high,118,122,125 accounting for 45.6% of the total health-care spend in 2018.126 This may be a factor in citizens taking a more pro-active role in personal health-care decisions, with vaccine refusal serving as an expression of personal freedoms, although undoubtedly other reasons play their part; data from a recent Wellcome Trust survey conducted in 2018 indicate that only 50% of the respondents considered vaccines to be effective, with only 29% considering vaccines to be safe.127 A high element of vaccine hesitancy and refusal by HCWs is also apparent, and this is compounded by a lack of awareness/knowledge, with a clear need for HCW education on vaccine benefits. Whether the considerable health-care reform ongoing in Ukraine leads to some improvement in confidence in the system remains uncertain.122
Framework analysis
Conclusions from our framework analysis reflecting the policy triangle of content, context and process for each country are summarized in Supplementary Table 1. The major findings from a wider perspective across all six countries are shown in Table 1. A number of broad themes emerge (summarized in Figure 4).
Table 1.
Framework analysis across countries
| Problem | Context | Policy implementation and required organizational mechanisms | Key observations |
|---|---|---|---|
| Decline in infant MCV coverage |
|
|
|
| Increasing disease burden in older age groups |
|
|
|
| Identify and reduce disease burden in hard-to-reach populations |
|
|
|
| Ensure agile response to changing epidemiology and outbreaks |
|
|
|
Figure 4.
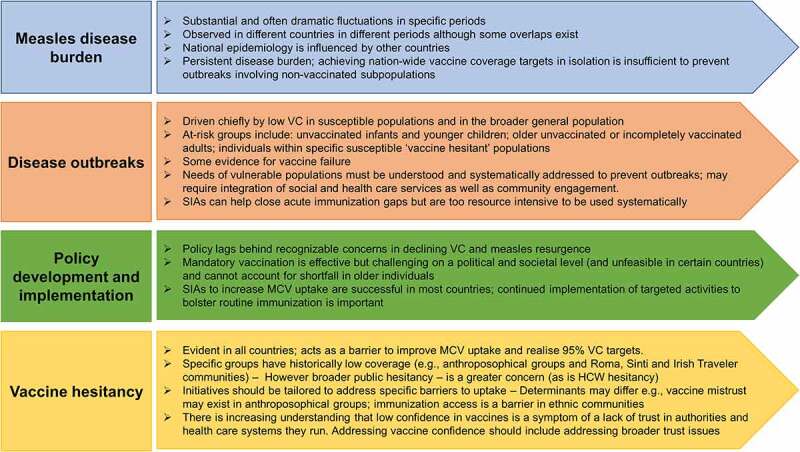
Emergent themes.
Semi-structured interviews
A total of nine key informant interviews were conducted between April and August 2020 (four with Italian experts and five from the UK), each lasting between 40 and 70 minutes. Informants represented a range of stakeholders; epidemiologists, physicians, and individuals involved in programatic policy and implementation, working chiefly within governmental public health institutions, and academic/industry representatives (Supplementary Table 2). Six key themes were apparent from thematic analysis (Table 2). In general, there was consistent agreement between both country experts on some aspects, e.g., the benefits of a centralized immunization framework (allied with effective regional implementation); value of coherent political commitment; and the need for closer public/community engagement and education to inform and address vaccine hesitancy or mistrust (especially in communities with specific unmet needs). However, there were clear differences in mandatory vaccination; while positively perceived by Italian respondents, UK respondents expressed reservations about acceptance (politically and by the general public). Most respondents considered that the ongoing COVID-19 pandemic provides an opportunity to refocus public health activities (and may reinforce the perceived value of outbreak control measures and immunization benefits). While greater trust in health-care systems may exist (in the short term), the longer term attitudes are less clear.
Table 2.
Key themes from semi-structured interviews
| Theme | Country | Role |
|---|---|---|
| Centralized immunization framework and political commitment | Italy |
|
| UK |
|
|
| Top-down vs bottom-up implementation and equity | Italy and UK |
|
| Incentives to vaccinate and mandatory vaccination | Italy |
|
| UK |
|
|
| Vaccine hesitancy as a symptom of declining trust | Italy and UK |
|
| Education | Italy and UK |
|
| Impact of COVID-19 on immunization attitudes | Italy and UK |
|
Discussion
Documenting temporal changes in measles epidemiology, vaccine uptake and policy, and outbreak response measures can help to provide some perspective on the structural interventions needed to achieve effective measles control (and ultimately elimination). Our observations highlight a diverse range of challenges that exist in developing robust policies and strategies to address measles disease burden in specific countries (or regions within countries). While each country has specific characteristics, a consistent pattern is that of previous complacency (as evident by often delayed responses to what would seem to be predictable outbreaks in most if not all countries) and a fragmented approach toward achieving the desired 95% vaccine uptake target, allied with often substantial and increasing elements of vaccine hesitancy (due to individual complacency, or lack of access or lack of confidence or overt mistrust) either within the general population or within specific communities. At a more macro level, there is a clear political dimension to immunization policy development and also a clear societal and often cultural dimension toward how vaccination measures are perceived and accessed by the general public.
Developing successful strategies need to take into account often complex factors. While outbreak response measures have been successful in limiting measles outbreaks in most countries, in isolation they do not address the underlying causes nor the evolving societal challenges, and additional strategies are necessary. Most countries we examined are taking positive actions, with a transition beyond a focus on infant immunization toward strategies that will also help close immunization gaps in adults and hard-to-reach subpopulations. Mandatory immunization has shown benefits in improving pediatric vaccine uptake in Italy; with first MMR dose at 24 months of age increasing from 87.3% in 2016 to 94.5% in 2019 and the corresponding second dose at 6 years increasing from 82.2% to 87.6%.128,129 Although impact data for Germany are not available, this measure is considered in generally favorable terms by German physicians.105 However, such mandates also provide a focus for public distrust and anti-vaccination sentiments, and for political and social reasons may seem unfeasible in some countries. Strengthening current childhood immunization programs along with SIAs and targeting susceptible populations, including individuals who missed out on previous MCV opportunities is essential. Success is reliant upon improving or restoring public confidence, through both broad and tailored community engagement. In this, HCW advocacy is clearly essential. Efforts must be made to provide education for physicians and other HCWs, to inform and address HCW hesitancy, and also to improve HCW measles vaccination to reduce nosocomial transmission. Increasing access by expanding vaccination opportunities, e.g., via mobile vaccination units and pharmacy-led immunization can also be effective.130
This study has some limitations. Only six European countries were included (and only two were evaluated using qualitative interviews). Perhaps more importantly, our study was conducted in 2020, in the initial phases of the COVID-19 pandemic, and our observations on measles epidemiology and immunization policy and implementation relate to the decade prior to the current COVID-19 pandemic. From a pragmatic perspective, awareness that immunization schedules and disease surveillance would be impacted by this pandemic, generally and within specific phases of the ongoing pandemic, made direct evaluation of the status in 2020 problematic (and beyond the broader objectives of the present study). From the epidemiologic perspective, the data we report across 2001–2019 (based on WHO surveillance data)16–21 could be considered reasonably robust, but it provides no context to the current epidemiological situation. While the full impact of the COVID-19 pandemic on measles immunization services (and the broader impact on routine vaccination delivery) remains uncertain, a conservative view in light of the obvious effect on health services would be that there has been some adverse impact, with reports of decline in MCV uptake in European countries with mandatory immunization,131–133 and throughout the pandemic concerns have been raised regarding future global measles resurgence.134,135 Vaccination hesitancy toward SARS-CoV2 vaccination also exists both in the general public, e.g., in the UK136 and Belgium, where attitudinal differences in French and Flemish communities also exist,137 and also in HCWs (as recently reported in Ukraine and Italy).124,138
While we do not wish to speculate on the impact of the ongoing COVID-19 pandemic upon specific measles policy and subsequent actions, the events and experiences of the pandemic will clearly inform and influence political and social attitudes toward immunization (in the broader sense). A plain language summary graphic which outlines the context, outcomes, and impact of this study is shown in Supplementary Figure 1.
Supplementary Material
Acknowledgments
The authors would like to thank Yasuko Vojtek Yoshino for support in performance and delivery of the literature review and social research. The authors would also wish to thank Business & Decision Life Sciences platform for editorial assistance and publication coordination, on behalf of GSK. Pierre-Paul Prevot coordinated publication development and editorial support. The authors also thank Iain O’Neill (freelance on behalf of GSK) for providing medical writing support.
Funding Statement
GlaxoSmithKline Biologicals SA funded this report and all costs related to the development and publication of this manuscript.
Authors’ contributions
IV conducted the initial literature review, interviews and framework analyses that informed the study report and subsequent manuscript, and developed the initial draft. All authors participated in subsequent manuscript development and review and approved the final submitted version.
Disclosure statement
IV is employed by the GSK group of companies and holds shares in the GSK group of companies. SP has received fees from Sanofi, Merck, Janssen, Inovio, Moderna, Astra Zeneca, Rational Vaccines, NTxBio, Seqirus, Codagenix, Vaxinnity, and Valneva outside the submitted work. HL has received grants from the GSK group of companies, Merck and Janssen and payment from the GSK group of companies for presentations outside the submitted work. IV, SP and HL declare no other financial and non-financial relationships and activities. PVD declares no financial and non-financial relationships and activities and no conflicts of interest.
Supplementary material
Supplemental data for this article can be accessed on the publisher’s website at https://doi.org/10.1080/21645515.2022.2031776
References
- 1.Perry RT, Gacic-Dobo M, Dabbagh A, Mulders MN, Strebel PM, Okwo-Bele JM, Rota PA, Goodson JL.. Global control and regional elimination of measles, 2000-2012. MMWR Morb Mortal Wkly Rep. 2014;63:103–15. [PMC free article] [PubMed] [Google Scholar]
- 2.Patel MK, Antoni S, Nedelec Y, Sodha S, Menning L, Ogbuanu IU, Gacic Dobo M. The changing global epidemiology of measles, 2013-2018. J Infect Dis. 2020;222:1117–28. doi: 10.1093/infdis/jiaa044. [DOI] [PubMed] [Google Scholar]
- 3.Zimmerman LA, Muscat M, Singh S, Ben Mamou M, Jankovic D, Datta S, Alexander JP, Goodson JL, O’Connor P. Progress toward measles elimination - European Region, 2009-2018. MMWR Morb Mortal Wkly Rep. 2019;68:396–401. doi: 10.15585/mmwr.mm6817a4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Khetsuriani N, Deshevoi S, Goel A, Spika J, Martin R, Emiroglu N. Supplementary immunization activities to achieve measles elimination: experience of the European Region. J Infect Dis. 2011;204(Suppl 1):S343–52. doi: 10.1093/infdis/jir074. [DOI] [PubMed] [Google Scholar]
- 5.Datta SS, O’Connor PM, Jankovic D, Muscat M, Ben Mamou MC, Singh S, Kaloumenos T, Reef S, Papania M, Butler R. Progress and challenges in measles and rubella elimination in the WHO European Region. Vaccine. 2018;36:5408–15. doi: 10.1016/j.vaccine.2017.06.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.ECDC . Surveillance Report. Measles. Annual Epidemiological Report for 2018. https://www.ecdc.europa.eu/en/publications-data/measles-annual-epidemiological-report-2018.
- 7.Bechini A, Boccalini S, Ninci A, Zanobini P, Sartor G, Bonaccorsi G, Grazzini M, Bonanni P. Childhood vaccination coverage in Europe: impact of different public health policies. Expert Rev Vaccines. 2019;18:693–701. doi: 10.1080/14760584.2019.1639502. [DOI] [PubMed] [Google Scholar]
- 8.Wilder-Smith AB, Qureshi K. Resurgence of measles in Europe: a systematic review on parental attitudes and beliefs of measles vaccine. J Epidemiol Glob Health. 2020;10:46–58. doi: 10.2991/jegh.k.191117.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.de Figueiredo A, Karafillakis E, and Larson HJ. State of Vaccine Confidence in the EU+UK 2020: report for the European Commission. Luxembourg: Publications Office of the European Union; 2020. https://ec.europa.eu/health/sites/default/files/vaccination/docs/2020_confidence_rep_en.pdf. [Google Scholar]
- 10.Bozzola E, Spina G, Russo R, Bozzola M, Corsello G, Villani A. Mandatory vaccinations in European countries, undocumented information, false news and the impact on vaccination uptake: the position of the Italian pediatric society. Ital J Pediatr. 2018;44:67. doi: 10.1186/s13052-018-0504-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Holzmann H, Wiedermann U. Mandatory vaccination: suited to enhance vaccination coverage in Europe? Euro Surveill. 2019;24:1900376. doi: 10.2807/1560-7917.ES.2019.24.26.1900376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Vaz OM, Ellingson MK, Weiss P, Jenness SM, Bardaji A, Bednarczyk RA, Omer SB. Mandatory Vaccination in Europe. Pediatrics. 2020;145:e20190620. doi: 10.1542/peds.2019-0620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Palinkas LA, Horwitz SM, Green CA, Wisdom JP, Duan N, Hoagwood K. Purposeful sampling for qualitative data collection and analysis in mixed method implementation research. Adm Policy Ment Health. 2015;42:533–44. doi: 10.1007/s10488-013-0528-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Nowell LS, Norris JM, White DE, and Moules NJ. Thematic analysis: striving to meet the trustworthiness criteria. Int J Qual Meth. 2017;16:1–13. doi: 10.1177/1609406917733847. [DOI] [Google Scholar]
- 15.Gilson Gwa L. Reforming the health sector in developing countries: the central role of policy analysis. Health Policy Plan. 1994;9:353–70. doi: 10.1093/heapol/9.4.353. [DOI] [PubMed] [Google Scholar]
- 16.WHO . WHO vaccine-preventable diseases: monitoring system. 2020 global summary. https://apps.who.int/immunization_monitoring/globalsummary.
- 17.Rodyna R. Measles situation in Ukraine during the period 2017-2019. Eur J Public Health. 2019;29. doi: 10.1093/eurpub/ckz186.496. [DOI] [Google Scholar]
- 18.Smiianov VA, Kurhanska VA, Smiianova OI. Measles outbreaks: they are preventable but keep progressing dangerously. Wiad Lek. 2019;72:2145–48. doi: 10.36740/WLek201911115. [DOI] [PubMed] [Google Scholar]
- 19.IFRC . Ukraine: measles outbreak DREF operation n° MDRUA009 Final Report (December 2019). https://reliefweb.int/report/ukraine/ukraine-measles-outbreak-dref-operation-n-mdrua009-final-report.
- 20.WHO-Europe . Measles and rubella country profile – Ukraine. 2019. [2013-2017]. https://www.euro.who.int/en/countries/ukraine/data-and-statistics/measles-and-rubella-country-profile-ukraine-2019.
- 21.WHO-Europe . Measles and rubella elimination country profile – Ukraine. 2020. [2014-2018]. https://www.euro.who.int/en/health-topics/disease-prevention/vaccines-and-immunization/publications/surveillance-and-data/measles-and-rubella-elimination-country-profiles/2020/measles-and-rubella-elimination-country-profile-ukraine-2020
- 22.ECDC . Measles: recommended vaccinations. ECDC. https://vaccine-schedule.ecdc.europa.eu/Scheduler/ByDisease?SelectedDiseaseId=8&SelectedCountryIdByDisease=−1. [Google Scholar]
- 23.Public-Health-England . The complete routine immunisation schedule from June 2020 (for infants born on or after 1 January 2020). https://www.gov.uk/government/publications/the-complete-routine-immunisation-schedule.
- 24.Rechel B, Richardson E, McKee M. The organization and delivery of vaccination services in the European Union. European Observatory on Health Systems and Policies. WHO Europe, 2020. 2018. https://apps.who.int/iris/handle/10665/330345. [Google Scholar]
- 25.Adamo G, Sturabotti G, Baccolini V, de Soccio P, Prencipe GP, Bella A, Magurano F, Iannazzo S, Villari P, Marzuillo C. Regional reports for the subnational monitoring of measles elimination in Italy and the identification of local barriers to the attainment of the elimination goal. PLoS One. 2018;13(10):e0205147. doi: 10.1371/journal.pone.0205147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Montalti M, Kawalec A, Leoni E, Dallolio L. Measles immunization policies and vaccination coverage in EU/EEA countries over the last decade. Vaccines (Basel). 2020;8(1):86. doi: 10.3390/vaccines8010086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.D’Ancona F, D’Amario C, Maraglino F, Rezza G, Iannazzo S. The law on compulsory vaccination in Italy: an update 2 years after the introduction. Euro Surveill. 2019;24(26). doi: 10.2807/1560-7917.ES.2019.24.26.1900371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Adamo G, Baccolini V, Massimi A, Barbato D, Cocchiara R, Di Paolo C, Mele A, Cianfanelli S, Angelozzi A, Castellani F, et al. Towards elimination of measles and rubella in Italy: progress and challenges. PLoS One. 2019;14(12):e0226513. doi: 10.1371/journal.pone.0226513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Magurano F, Fortuna C, Baggieri M, Filia A, Benedetti E, Bucci P, Marchi A, Nicoletti L. Molecular epidemiology of measles virus in Italy during 2008. Ann Ist Super Sanita. 2013;49:50–55. doi: 10.4415/ANN_13_01_09. [DOI] [PubMed] [Google Scholar]
- 30.Filia A, Bella A, Rota M, Tavilla A, Magurano F, Baggieri M, Nicoletti L, Iannazzo S, Pompa MG, Declich S. Analysis of national measles surveillance data in Italy from October 2010 to December 2011 and priorities for reaching the 2015 measles elimination goal. Euro Surveill. 2013;18(20):20480. doi: 10.2807/ese.18.20.20480-en. [DOI] [PubMed] [Google Scholar]
- 31.Filia A, Bella A, Del Manso M, Baggieri M, Magurano F, Rota MC. Ongoing outbreak with well over 4,000 measles cases in Italy from January to end August 2017 - what is making elimination so difficult? Euro Surveill. 2017;22:30614. doi: 10.2807/1560-7917.ES.2017.22.37.30614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Filia A, Curtale F, Kreidl P, Morosetti G, Nicoletti L, Perrelli F, Mantovani J, Campus D, Rossi G, Sanna MC, et al. Cluster of measles cases in the Roma/Sinti population, Italy, June-September 2006. Euro Surveill. 2006;11:E061012 2. doi: 10.2807/esw.11.41.03062-en. [DOI] [PubMed] [Google Scholar]
- 33.Curtale F, Perrelli F, Mantovani J, Ciofi Degli Atti M, Filia A, Nicoletti L, Magurano F, Borgia P, Di Lallo D. Description of two measles outbreaks in the Lazio Region, Italy (2006-2007). Importance of pockets of low vaccine coverage in sustaining the infection. BMC Infect Dis. 2010;10(1):62. doi: 10.1186/1471-2334-10-62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Filia A, Amendola A, Faccini M, Del Manso M, Senatore S, Bianchi S, Borrini BM, Ciampelli A, Tanzi E, Filipponi MT, et al. Outbreak of a new measles B3 variant in the Roma/Sinti population with transmission in the nosocomial setting, Italy, November 2015 to April 2016. Euro Surveill. 2016;21(20):30235. doi: 10.2807/1560-7917.ES.2016.21.20.30235. [DOI] [PubMed] [Google Scholar]
- 35.Monasta L, Knowles A. Letter to the editor: outbreak of a new measles B3 variant in the Roma/Sinti population with transmission in the nosocomial setting, Italy, November 2015 to April 2016. Euro Surveill. 2016;21. doi: 10.2807/1560-7917.ES.2016.21.27.30275. [DOI] [PubMed] [Google Scholar]
- 36.Filia A, Faccini M, Amendola A, and Magurano F. authors of the original a. Authors’ reply: outbreak of a new measles B3 variant in the Roma/Sinti population with transmission in the nosocomial setting, Italy, November 2015 to April 2016. Euro Surveill. 2016;21(27):30276. doi: 10.2807/1560-7917.ES.2016.21.27.30276. [DOI] [PubMed] [Google Scholar]
- 37.Amendola A, Bianchi S, Frati ER, Ciceri G, Faccini M, Senatore S, Colzani D, Lamberti A, Baggieri M, Cereda D. Ongoing large measles outbreak with nosocomial transmission in Milan, northern Italy, March-August 2017. Euro Surveill. 2017;22:30596. doi: 10.2807/1560-7917.ES.2017.22.33.30596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Baccolini V, Sindoni A, Adamo G, Rosso A, Massimi A, Bella A, Filia A, Magurano F, Marzuillo C, Villari P, et al. Measles among healthcare workers in Italy: is it time to act? Hum Vaccin Immunother. 2020;16(11):2618–27. doi: 10.1080/21645515.2020.1737458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Signorelli C, Odone A, Cella P, Iannazzo S, D’Ancona F, Guerra R. Infant immunization coverage in Italy (2000-2016). Ann Ist Super Sanita. 2017;53:231–37. doi: 10.4415/ANN_17_03_09. [DOI] [PubMed] [Google Scholar]
- 40.Siani A. Measles outbreaks in Italy: a paradigm of the re-emergence of vaccine-preventable diseases in developed countries. Prev Med. 2019;121:99–104. doi: 10.1016/j.ypmed.2019.02.011. [DOI] [PubMed] [Google Scholar]
- 41.Signorelli C, Odone A. Four Italian experiences on vaccination policies: results and lessons. Ann Ig. 2019;31:36–44. doi: 10.7416/ai.2019.2275. [DOI] [PubMed] [Google Scholar]
- 42.Tavoschi L, Quattrone F, De Vita E, Lopalco PL. Impact of mandatory law on vaccine hesitancy spectrum: the case of measles vaccine catch-up activities in Tuscany, Italy. Vaccine. 2019;37:7201–02. doi: 10.1016/j.vaccine.2019.09.092. [DOI] [PubMed] [Google Scholar]
- 43.Signorelli C, Iannazzo S, Odone A. The imperative of vaccination put into practice. Lancet Infect Dis. 2018;18:26–27. doi: 10.1016/S1473-3099(17)30696-5. [DOI] [PubMed] [Google Scholar]
- 44.Odone A, Bucci D, Croci R, Ricco M, Affanni P, Signorelli C. Vaccine hesitancy in COVID-19 times. An update from Italy before flu season starts. Acta Biomed. 2020;91:e2020031. doi: 10.23750/abm.v91i3.10549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Fadda M, Galimberti E, Carraro V, Schulz PJ. What are parents’ perspectives on psychological empowerment in the MMR vaccination decision? A focus group study. BMJ Open. 2016;6:e010773. doi: 10.1136/bmjopen-2015-010773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Facciola A, Visalli G, Orlando A, Bertuccio MP, Spataro P, Squeri R, Picerno I, Di Pietro A. Vaccine hesitancy: an overview on parents’ opinions about vaccination and possible reasons of vaccine refusal. J Public Health Res. 2019;8:1436. doi: 10.4081/jphr.2019.1436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Lancella L, Di Camillo C, Vittucci AC, Boccuzzi E, Bozzola E, Villani A. Measles lessons in an anti-vaccination era: public health is a social duty, not a political option. Ital J Pediatr. 2017;43:102. doi: 10.1186/s13052-017-0420-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Dalla Zuanna T, Del Manso M, Giambi C, Riccardo F, Bella A, Caporali MG, Dente M, Declich S. Immunization Offer Targeting Migrants: policies and Practices in Italy. Int J Environ Res Public Health. 2018;15:968. doi: 10.3390/ijerph15050968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Mipatrini D, Stefanelli P, Severoni S, Rezza G. Vaccinations in migrants and refugees: a challenge for European health systems. A systematic review of current scientific evidence. Pathog Glob Health. 2017;111:59–68. doi: 10.1080/20477724.2017.1281374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Ceccarelli G, Vita S, Riva E, Cella E, Lopalco M, Antonelli F, De Cesaris M, Fogolari M, Dicuonzo G, Ciccozzi M. Susceptibility to measles in migrant population: implication for policy makers. J Travel Med. 2018;25(1):1–5. doi: 10.1093/jtm/tax080. [DOI] [PubMed] [Google Scholar]
- 51.Wise J. Meningitis B vaccine to be introduced in UK after U turn on its cost effectiveness. BMJ. 2014;348:g2327. doi: 10.1136/bmj.g2327. [DOI] [PubMed] [Google Scholar]
- 52.Ramsay ME. Measles: the legacy of low vaccine coverage. Arch Dis Child. 2013;98:752–54. doi: 10.1136/archdischild-2013-304292. [DOI] [PubMed] [Google Scholar]
- 53.Edelstein M, White J, Bukasa A, Saliba V, Ramsay M. Triangulation of measles vaccination data in the United Kingdom of Great Britain and Northern Ireland. Bull World Health Organ. 2019;97:754–63. doi: 10.2471/BLT.18.229138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Public-Health-England . Measles and rubella elimination UK strategy. London, UK: Public Health England; 2019. https://www.gov.uk/government/publications/measles-and-rubella-elimination-uk-strategy. [Google Scholar]
- 55.Ghebrehewet S, Hayhurst G, Keenan A, Moore H. Outbreak of measles in Central and Eastern Cheshire, UK, October 2008-February 2009. Epidemiol Infect. 2013;141:1849–56. doi: 10.1017/S0950268812002300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Vivancos R, Keenan A, Farmer S, Atkinson J, Coffey E, Dardamissis E, Dillon J, Drew RJ, Fallon M, Huyton R, et al. An ongoing large outbreak of measles in Merseyside, England, January to June 2012. Euro Surveill. 2012;17:20226. doi: 10.2807/ese.17.31.20234-en. [DOI] [PubMed] [Google Scholar]
- 57.Keenan A, Ghebrehewet S, Vivancos R, Seddon D, MacPherson P, Hungerford D. Measles outbreaks in the UK, is it when and where, rather than if? A database cohort study of childhood population susceptibility in Liverpool, UK. BMJ Open. 2017;7(3):e014106. doi: 10.1136/bmjopen-2016-014106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Pegorie M, Shankar K, Welfare WS, Wilson RW, Khiroya C, Munslow G, Fiefield D, Bothra V, McCann R. Measles outbreak in Greater Manchester, England, October 2012 to September 2013: epidemiology and control. Euro Surveill. 2014;19(49):20982. doi: 10.2807/1560-7917.ES2014.19.49.20982. [DOI] [PubMed] [Google Scholar]
- 59.Baugh V, Figueroa J, Bosanquet J, Kemsley P, Addiman S, Turbitt D. Ongoing measles outbreak in Orthodox Jewish community, London, UK. Emerg Infect Dis. 2013;19(10):1707–09. doi: 10.3201/eid1910.130258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Cohuet S, Bukasa A, Heathcock R, White J, Brown K, Ramsay M, Fraser G. A measles outbreak in the Irish traveller ethnic group after attending a funeral in England, March-June 2007. Epidemiol Infect. 2009;137:1759–65. doi: 10.1017/S0950268809002714. [DOI] [PubMed] [Google Scholar]
- 61.Maduma-Butshe A, McCarthy N. The burden and impact of measles among the Gypsy-Traveller communities, Thames Valley, 2006-09. J Public Health (Oxf). 2013;35:27–31. doi: 10.1093/pubmed/fds052. [DOI] [PubMed] [Google Scholar]
- 62.Bell S, Saliba V, Ramsay M, Mounier-Jack S. What have we learnt from measles outbreaks in 3 English cities? A qualitative exploration of factors influencing vaccination uptake in Romanian and Roma Romanian communities. BMC Public Health. 2020;20:381. doi: 10.1186/s12889-020-8454-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Currie J, Davies L, McCarthy J, Perry M, Moore C, Cottrell S, Bowley M, Williams C, Shankar AG, Stiff R. Measles outbreak linked to European B3 outbreaks, Wales, United Kingdom, 2017. Euro Surveill. 2017;22:17–00673. doi: 10.2807/1560-7917.ES.2017.22.42.17-00673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Le Menach A, Boxall N, Amirthalingam G, Maddock L, Balasegaram S, Mindlin M. Increased measles-mumps-rubella (MMR) vaccine uptake in the context of a targeted immunisation campaign during a measles outbreak in a vaccine-reluctant community in England. Vaccine. 2014;32:1147–52. doi: 10.1016/j.vaccine.2014.01.002. [DOI] [PubMed] [Google Scholar]
- 65.Hungerford D, Macpherson P, Farmer S, Ghebrehewet S, Seddon D, Vivancos R, Keenan A. Effect of socioeconomic deprivation on uptake of measles, mumps and rubella vaccination in Liverpool, UK over 16 years: a longitudinal ecological study. Epidemiol Infect. 2016;144:1201–11. doi: 10.1017/S0950268815002599. [DOI] [PubMed] [Google Scholar]
- 66.Letley L, Rew V, Ahmed R, Habersaat KB, Paterson P, Chantler T, Saavedra-Campos M, Butler R. Tailoring immunisation programmes: using behavioural insights to identify barriers and enablers to childhood immunisations in a Jewish community in London, UK. Vaccine. 2018;36(31):4687–92. doi: 10.1016/j.vaccine.2018.06.028. [DOI] [PubMed] [Google Scholar]
- 67.McHale P, Keenan A, Ghebrehewet S. Reasons for measles cases not being vaccinated with MMR: investigation into parents’ and carers’ views following a large measles outbreak. Epidemiol Infect. 2016;144:870–75. doi: 10.1017/S0950268815001909. [DOI] [PubMed] [Google Scholar]
- 68.Edelstein M, Muller M, Ladhani S, Yarwood J, Salathe M, Ramsay M. Keep calm and carry on vaccinating: is anti-vaccination sentiment contributing to declining vaccine coverage in England? Vaccine. 2020;38:5297–304. doi: 10.1016/j.vaccine.2020.05.082. [DOI] [PubMed] [Google Scholar]
- 69.Draeger E, Bedford HE, Elliman DAC. Should measles vaccination be compulsory? BMJ. 2019;365:l2359. doi: 10.1136/bmj.l2359. [DOI] [PubMed] [Google Scholar]
- 70.Griffith R. Can the courts order that a child be immunised? Br J Nurs. 2016;25(19):1076–77. doi: 10.12968/bjon.2016.25.19.1076. [DOI] [PubMed] [Google Scholar]
- 71.Belgian Health Care Knowledge Centre (KCE) . Performance of the Belgian health system – report 2019. https://www.healthybelgium.be/metadata/hspa/p1_p2_p3.pdf.
- 72.Lernout T, Kissling E, Hutse V, De Schrijver K, Top G. An outbreak of measles in orthodox Jewish communities in Antwerp, Belgium, 2007-2008: different reasons for accumulation of susceptibles. Euro Surveill. 2009;14(2):19087. doi: 10.2807/ese.14.02.19087-en. [DOI] [PubMed] [Google Scholar]
- 73.Asnong C, Van Herck K, Lernout T, Theeten H, Van Damme P. Lessons learned from a measles outbreak in Antwerp, Belgium 2007-2008. Pediatr Infect Dis J. 2011;30(4):343–45. doi: 10.1097/INF.0b013e3181fbf5b7. [DOI] [PubMed] [Google Scholar]
- 74.Braeye T, Sabbe M, Hutse V, Flipse W, Godderis L, Top G. Obstacles in measles elimination: an in-depth description of a measles outbreak in Ghent, Belgium, spring 2011. Arch Public Health. 2013;71(1):17. doi: 10.1186/0778-7367-71-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Grammens T, Schirvel C, Leenen S, Shodu N, Hutse V, Mendes da Costa E, Sabbe M. Ongoing measles outbreak in Wallonia, Belgium, December 2016 to March 2017: characteristics and challenges. Euro Surveill. 2017;22:30524. doi: 10.2807/1560-7917.ES.2017.22.17.30524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Cornelissen L, Grammens T, Leenen S, Schirvel C, Hutse V, Demeester R, Swennen B, Asikainen T, Wyndham-Thomas C. High number of hospitalisations and non-classical presentations: lessons learned from a measles outbreak in 2017, Belgium. Epidemiol Infect. 2020;148:e35. doi: 10.1017/S0950268820000278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Robert E, Dramaix M, Swennen B. Vaccination coverage for infants: cross-sectional studies in two regions of Belgium. Biomed Res Int. 2014;2014:838907. doi: 10.1155/2014/838907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Gobert C, Semaille P, Van der Schueren T, Verger P, Dauby N. Prevalence and Determinants of Vaccine Hesitancy and Vaccines Recommendation Discrepancies among General Practitioners in French-Speaking Parts of Belgium. Vaccines (Basel). 2021;9. doi: 10.3390/vaccines9070771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Hellenbrand W, Siedler A, Tischer A, Meyer C, Reiter S, Rasch G, Teichmann D, Santibanez S, Altmann D, Claus H. Progress toward measles elimination in Germany. J Infect Dis. 2003;187(Suppl 1):S208–16. doi: 10.1086/368046. [DOI] [PubMed] [Google Scholar]
- 80.Takla A, Wichmann O, Rieck T, Matysiak-Klose D. Measles incidence and reporting trends in Germany, 2007-2011. Bull World Health Organ. 2014;92:742–49. doi: 10.2471/BLT.13.135145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Siedler A, Mankertz A, Feil F, Ahlemeyer G, Hornig A, Kirchner M, Beyrer K, Dreesman J, Scharkus S, Marcic A, et al. Closer to the goal: efforts in measles elimination in Germany 2010. J Infect Dis. 2011;204(Suppl 1):S373–80. doi: 10.1093/infdis/jir068. [DOI] [PubMed] [Google Scholar]
- 82.Sanftenberg L, Schrors HJ, Schelling J. Influences on immunization rates: vaccination coverage of mumps, measles, rubella and varicella before and after the STIKO intervention 2011 - A retrospective study. Vaccine. 2016;34:3938–41. doi: 10.1016/j.vaccine.2016.06.027. [DOI] [PubMed] [Google Scholar]
- 83.Storr C, Sanftenberg L, Schelling J, Heininger U, Schneider A. Measles Status-Barriers to Vaccination and Strategies for Overcoming Them. Dtsch Arztebl Int. 2018;115:723–30. doi: 10.3238/arztebl.2018.0723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Siedler A, Rieck T, Reuss A, Walter D, Poggensee G, Poethko-Muller C, Reiter S. Estimating vaccination coverage in the absence of immunisation registers–the German experience. Euro Surveill. 2012;17:20152. doi: 10.2807/ese.17.17.20152-en. [DOI] [PubMed] [Google Scholar]
- 85.Damm O, Witte J, Wetzka S, Prosser C, Braun S, Welte R, Greiner W. Epidemiology and economic burden of measles, mumps, pertussis, and varicella in Germany: a systematic review. Int J Public Health. 2016;61(7):847–60. doi: 10.1007/s00038-016-0842-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Hagemann C, Streng A, Kraemer A, Liese JG. Heterogeneity in coverage for measles and varicella vaccination in toddlers - analysis of factors influencing parental acceptance. BMC Public Health. 2017;17:724. doi: 10.1186/s12889-017-4725-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.van Treeck U, Wichmann O. Measles outbreak in Germany: update. Euro Surveill. 2006;11:E060413 1. doi: 10.2807/esw.11.15.02939-en. [DOI] [PubMed] [Google Scholar]
- 88.van Treeck U. Measles outbreak in Germany: over 1000 cases now reported in Nordrhein Westfalen. Euro Surveill. 2006;11:E060511 1. doi: 10.2807/esw.11.19.02955-en. [DOI] [PubMed] [Google Scholar]
- 89.Wichmann O, Hellenbrand W, Sagebiel D, Santibanez S, Ahlemeyer G, Vogt G, Siedler A, van Treeck U. Large measles outbreak at a German public school, 2006. Pediatr Infect Dis J. 2007;26:782–86. doi: 10.1097/INF.0b013e318060aca1. [DOI] [PubMed] [Google Scholar]
- 90.Bernard H, Santibanez S, Siedler A, Ludwig MS, Fischer R, Hautmann W. An outbreak of measles in Lower Bavaria, Germany, January-June 2007. Euro Surveill. 2007;12:E071004 1. doi: 10.2807/esw.12.40.03278-en. [DOI] [PubMed] [Google Scholar]
- 91.Bernard H, Fischer R, Wild F. Ongoing measles outbreak in southern Bavaria, Germany. Euro Surveill. 2008;13:8002. doi: 10.2807/ese.13.01.08002-en. [DOI] [PubMed] [Google Scholar]
- 92.Schmid D, Holzmann H, Abele S, Kasper S, Konig S, Meusburger S, Hrabcik H, Luckner-Hornischer A, Bechter E, DeMartin A. An ongoing multi-state outbreak of measles linked to non-immune anthroposophic communities in Austria, Germany, and Norway, March-April 2008. Euro Surveill. 2008;13:18838. doi: 10.2807/ese.13.16.18838-en. [DOI] [PubMed] [Google Scholar]
- 93.Wadl M, Siedler A, Kramer W, Haindl ME, Gebrande S, Krenn-Lanzl I, Mankertz A, Hautmann W. Measles transmission from an anthroposophic community to the general population, Germany 2008. BMC Public Health. 2011;11(1):474. doi: 10.1186/1471-2458-11-474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Batzing-Feigenbaum J, Pruckner U, Beyer A, Sinn G, Dinter A, Mankertz A, Siedler A, Schubert A, Suckau M. Spotlight on measles 2010: preliminary report of an ongoing measles outbreak in a subpopulation with low vaccination coverage in Berlin, Germany, January-March 2010. Euro Surveill. 2010;15(13):19527. doi: 10.2807/ese.15.13.19527-en. [DOI] [PubMed] [Google Scholar]
- 95.Roggendorf H, Mankertz A, Kundt R, Roggendorf M. Spotlight on measles 2010: measles outbreak in a mainly unvaccinated community in Essen, Germany, March-June 2010. Euro Surveill. 2010;15:19605. doi: 10.2807/ese.15.26.19605-en. [DOI] [PubMed] [Google Scholar]
- 96.Roggendorf H, Santibanez S, Mankertz A, van Treeck U, Roggendorf M. Two consecutive measles outbreaks with genotypes D8 and D4 in two mainly unvaccinated communities in Germany. Med Microbiol Immunol. 2012;201:349–55. doi: 10.1007/s00430-012-0240-7. [DOI] [PubMed] [Google Scholar]
- 97.Gillesberg Lassen S, Schuster M, Stemmler M, Steinmuller A, Matysiak-Klose D, Mankertz A, Santibanez S, Wichmann O, Falkenhorst G. Measles outbreak spreading from the community to an anthroposophic school, Berlin, 2011. Epidemiol Infect. 2014;142:789–96. doi: 10.1017/S0950268813001398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Mankertz A, Mihneva Z, Gold H, Baumgarte S, Baillot A, Helble R, Roggendorf H, Bosevska G, Nedeljkovic J, Makowka A. Spread of measles virus D4-Hamburg, Europe, 2008-2011. Emerg Infect Dis. 2011;17:1396–401. doi: 10.3201/eid1708.101994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Hegasy G, Katzner K, Helle M, Mankertz A, Baumgarte S, Wille A, Fell G. Description of measles D4-Hamburg outbreak in Hamburg, Germany, December 2008 to June 2009, which disproportionally affected a local Roma community. Euro Surveill. 2012;17(24):20194. doi: 10.2807/ese.17.24.20194-en. [DOI] [PubMed] [Google Scholar]
- 100.Werber D, Hoffmann A, Santibanez S, Mankertz A, Sagebiel D. Large measles outbreak introduced by asylum seekers and spread among the insufficiently vaccinated resident population, Berlin, October 2014 to August 2015. Euro Surveill. 2017;22:30599. doi: 10.2807/1560-7917.ES.2017.22.34.30599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Bitzegeio J, Majowicz S, Matysiak-Klose D, Sagebiel D, Werber D. Estimating age-specific vaccine effectiveness using data from a large measles outbreak in Berlin, Germany, 2014/15: evidence for waning immunity. Euro Surveill. 2019;24:1800529. doi: 10.2807/1560-7917.ES.2019.24.17.1800529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Muscat M, Marinova L, Mankertz A, Gatcheva N, Mihneva Z, Santibanez S, Kunchev A, Filipova R, Kojouharova M. The measles outbreak in Bulgaria, 2009-2011: an epidemiological assessment and lessons learnt. Euro Surveill. 2016;21:30152. doi: 10.2807/1560-7917.ES.2016.21.9.30152. [DOI] [PubMed] [Google Scholar]
- 103.Pfaff G. Elimination of measles and rubella in Germany: progress and hindrances. Bundesgesundheitsblatt Gesundheitsforschung Gesundheitsschutz. 2013;56:1222–24. doi: 10.1007/s00103-013-1803-5. [DOI] [PubMed] [Google Scholar]
- 104.Schroder-Back P, Martakis K. Counterpoint: should childhood vaccination against measles be a mandatory requirement for attending school? No. Chest. 2015;148:854–56. doi: 10.1378/chest.15-1162. [DOI] [PubMed] [Google Scholar]
- 105.Neufeind J, Betsch C, Zylka-Menhorn V, Wichmann O. Determinants of physician attitudes towards the new selective measles vaccine mandate in Germany. BMC Public Health. 2021;21:566. doi: 10.1186/s12889-021-10563-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Dascalu S. Measles Epidemics in Romania: lessons for Public Health and Future Policy. Front Public Health. 2019;7:98. doi: 10.3389/fpubh.2019.00098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Pistol A, Hennessey K, Pitigoi D, Ion-Nedelcu N, Lupulescu E, Walls L, Bellini W, Strebel P. Progress toward measles elimination in Romania after a mass vaccination campaign and implementation of enhanced measles surveillance. J Infect Dis. 2003;187(Suppl 1):S217–22. doi: 10.1086/368228. [DOI] [PubMed] [Google Scholar]
- 108.Ion-Nedelcu N, Craciun D, Pitigoi D, Popa M, Hennessey K, Roure C, Aston R, Zimmermann G, Pelly M, Gay N, et al. Measles elimination: a mass immunization campaign in Romania. Am J Public Health. 2001;91:1042–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Habersaat KB, Pistol A, Stanescu A, Hewitt C, Grbic M, Butu C, Jackson C. Measles outbreak in Romania: understanding factors related to suboptimal vaccination uptake. Eur J Public Health. 2020;30(5):986–92. doi: 10.1093/eurpub/ckaa079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Kriss JL, Stanescu A, Pistol A, Butu C, Goodson JL. The World Health Organization measles programmatic risk assessment Tool-Romania, 2015. Risk Anal. 2017;37:1096–107. doi: 10.1111/risa.12669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.ECDC . Rapid risk assessment: ongoing outbreak of measles in Romania, risk of spread and epidemiological situation in EU/EEA countries. 2017. Mar 3. https://www.ecdc.europa.eu/en/publications-data/rapid-risk-assessment-ongoing-outbreak-measles-romania-risk-spread-and.
- 112.Lazar M, Stanescu A, Penedos AR, Pistol A. Characterisation of measles after the introduction of the combined measles-mumps-rubella (MMR) vaccine in 2004 with focus on the laboratory data, 2016 to 2019 outbreak, Romania. Euro Surveill. 2019;24:1900041. doi: 10.2807/1560-7917.ES.2019.24.29.1900041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Pitigoi D, Sandulescu O, Craciun MD, Draganescu A, Jugulete G, Streinu-Cercel A, Vișan A, Rîciu C, Rafila A, Aramă V. Measles in Romania - clinical and epidemiological characteristics of hospitalized measles cases during the first three years of the 2016-ongoing epidemic. Virulence. 2020;11:686–94. doi: 10.1080/21505594.2020.1771948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Miko D, Costache C, Colosi HA, Neculicioiu V, Colosi IA. Qualitative Assessment of Vaccine Hesitancy in Romania. Medicina (Kaunas). 2019;55:282. doi: 10.3390/medicina55060282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Mokhort H, Kovalchuk A, Sokolovska O, Higgs S. Contribution of Vaccination to the Reduction of Infectious Mortality in Ukraine in the Second Half of the 20(th) and Early 21(st) Century: a Comparative Population-Based Study of the Dynamics and Structure of Infectious Mortality and Incidence. Viral Immunol. 2018;31:695–707. doi: 10.1089/vim.2018.0054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Velicko I, Muller LL, Pebody R, Gergonne B, Aidyralieva C, Kostiuchenko N, Spika JS, et al. Nationwide measles epidemic in Ukraine: the effect of low vaccine effectiveness. Vaccine. 2008;26:6980–85. doi: 10.1016/j.vaccine.2008.09.012. [DOI] [PubMed] [Google Scholar]
- 117.Spika JS, Aidyralieva C, Mukharskaya L, Kostyuchenko NN, Mulders M, Lipskaya G, Emiroglu N. Measles outbreak in the Ukraine, 2005-2006. Euro Surveill. 2006;11:E060309 1. doi: 10.2807/esw.11.10.02918-en. [DOI] [PubMed] [Google Scholar]
- 118.Bazylevych M. Vaccination campaigns in postsocialist Ukraine: health care providers navigating uncertainty. Med Anthropol Q. 2011;25:436–56. doi: 10.1111/j.1548-1387.2011.01179.x. [DOI] [PubMed] [Google Scholar]
- 119.Daragan GM, Krushinska TY, Stepanskiy DO, Demchyshyna IV, Kolesnikova IP. Topical issues of vaccination and epidemiological surveillance over measles and rubella in Ukraine. Medychni Perspektyvy. 2018;23:38–43. doi: 10.26641/2307-0404.2018.1(part1).127206. [DOI] [Google Scholar]
- 120.Smiianov VA, Zaitseva HS, Kurganskaya VA, Dyachenko AG, Zbarazhskyi VP, Smiianov YV, Pilipec OA. Vaccination coverage rates and the incidence of vaccine preventable diseases among children in sumy region of Ukraine. Wiad Lek. 2019;72:255–60. doi: 10.36740/WLek201902121. [DOI] [PubMed] [Google Scholar]
- 121.Koniushevska AA, Parkhomenko TA, Sharunova MV, Kazantsev AB, Yakovenko DV. Epidemiology and features of the measles course in children during the outbreak of 2018–2019 in the city of Mariupol. Regul Mech Biosyst. 2020;11:74–81. doi: 10.15421/022010. [DOI] [Google Scholar]
- 122.Romaniuk P, Semigina T. Ukrainian health care system and its chances for successful transition from Soviet legacies. Global Health. 2018;14:116. doi: 10.1186/s12992-018-0439-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Colborne M. Tackling Pharma corruption in Ukraine. CMAJ. 2017;189:E1101–E2. doi: 10.1503/cmaj.1095454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Holt E. COVID-19 vaccination in Ukraine. Lancet Infect Dis. 2021;21:462. doi: 10.1016/S1473-3099(21)00156-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Lekhan V, Rudiy V, Shevchenko M, Nitzan Kaluski D, Richardson E. Ukraine: health system review. Health Syst Transit. 2015;17:1–154. [PubMed] [Google Scholar]
- 126.WHO . Ukraine health reform: leapfrogging to modern health system. 2019. https://www.euro.who.int/__data/assets/pdf_file/0005/370850/pollab-ukraine-eng.pdf.
- 127.Wellcome-Trust . Wellcome Global Monitor 2018. Chapter 5: attitudes to vaccines. https://wellcome.org/reports/wellcome-global-monitor/2018/chapter-5-attitudes-vaccines.
- 128.Odone A, Dallagiacoma G, Frascella B, Signorelli C, Leask J. Current understandings of the impact of mandatory vaccination laws in Europe. Expert Rev Vaccines. 2021;20:559–75. doi: 10.1080/14760584.2021.1912603. [DOI] [PubMed] [Google Scholar]
- 129.Sabbatucci M, Odone A, Signorelli C, Siddu A, Maraglino F, Rezza G. Improved temporal trends of vaccination coverage rates in childhood after the mandatory vaccination act, Italy 2014–2019. J Clin Med. 2021;10:2540. doi: 10.3390/jcm10122540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Ecarnot F, Crepaldi G, Juvin P, Grabenstein J, Del Giudice G, Tan L, O’Dwyer S, Esposito S, Bosch X, Gavazzi G. Pharmacy-based interventions to increase vaccine uptake: report of a multidisciplinary stakeholders meeting. BMC Public Health. 2019;19:1698. doi: 10.1186/s12889-019-8044-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Bechini A, Garamella G, Giammarco B, Zanella B, Flori V, Bonanni P, Boccalini S. Paediatric activities and adherence to vaccinations during the COVID-19 epidemic period in Tuscany, Italy: a survey of paediatricians. J Prev Med Hyg. 2020;61:E125–E9. doi: 10.15167/2421-4248/jpmh2020.61.2.1626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Chiappini E, Parigi S, Galli L, Licari A, Brambilla I, Angela Tosca M, Ciprandi G, Marseglia G. Impact that the COVID-19 pandemic on routine childhood vaccinations and challenges ahead: a narrative review. Acta Paediatr. 2021;110(9):2529–35. doi: 10.1111/apa.15949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Taine M, Offredo L, Drouin J, Toubiana J, Weill A, Zureik M, Dray-Spira R. Mandatory Infant Vaccinations in France During the COVID-19 Pandemic in 2020. Front Pediatr. 2021;9:666848. doi: 10.3389/fped.2021.666848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Guha-Sapir D, Moitinho de Almeida M, Keita M, Greenough G, Bendavid E. COVID-19 policies: remember measles. Science. 2020;369:261. doi: 10.1126/science.abc8637. [DOI] [PubMed] [Google Scholar]
- 135.Durrheim DN, Andrus JK, Tabassum S, Bashour H, Githanga D, Pfaff G. A dangerous measles future looms beyond the COVID-19 pandemic. Nat Med. 2021;27:360–61. doi: 10.1038/s41591-021-01237-5. [DOI] [PubMed] [Google Scholar]
- 136.Freeman D, Loe BS, Chadwick A, Vaccari C, Waite F, Rosebrock L, Jenner L, Petit A, Lewandowsky S, Vanderslott S, et al. COVID-19 vaccine hesitancy in the UK: the Oxford coronavirus explanations, attitudes, and narratives survey (Oceans) II. Psychol Med. 2020;1–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.ICense . iCense reveals the reasons of vaccination hesitancy in the Belgian population. 2021. Jun 27. https://www.icense.org/blog–press/icense-reveals-the-reasons-of-vaccination-hesitancy-in-the-belgian-population.
- 138.Di Gennaro F, Murri R, Segala FV, Cerruti L, Abdulle A, Saracino A, Bavaro DF, Fantoni M. Attitudes towards Anti-SARS-CoV2 vaccination among healthcare workers: results from a National Survey in Italy. Viruses. 2021;13:371. doi: 10.3390/v13030371. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


