Figure 4.
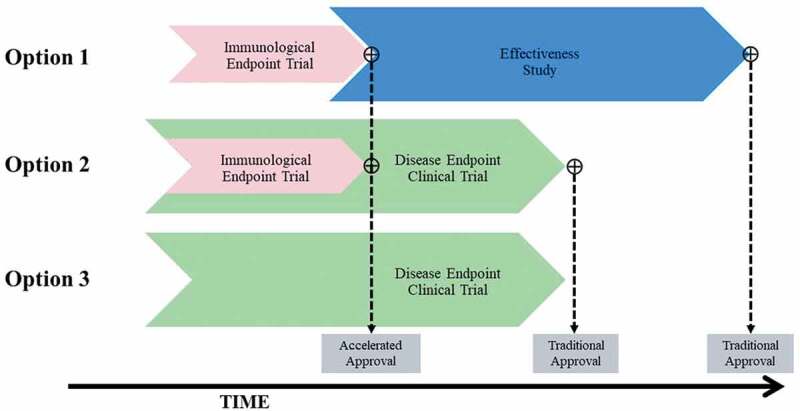
Overview of potential approaches to licensure of a maternal GBS6 vaccine. Effectiveness study refers to a clinical endpoint trial that is conducted under real-world settings after vaccine licensure. Disease endpoint clinical trial refers to an efficacy trial with GBS disease as the primary study endpoint. Accelerated approval is not applicable to Option 3.
