FIGURE 4.
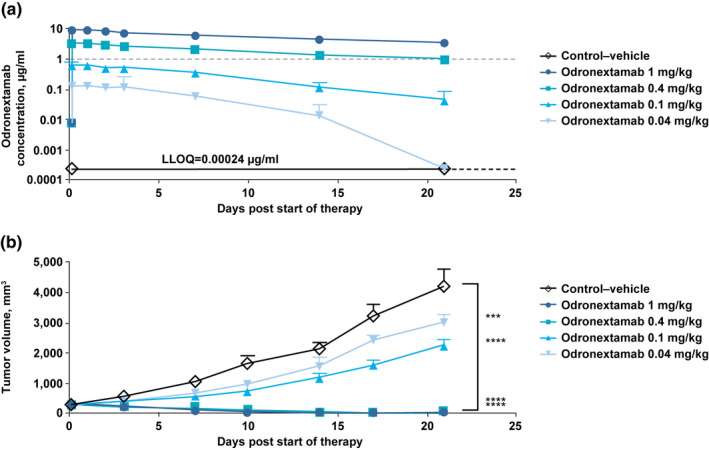
Odronextamab exposure (a) and antitumor effects (b) in immunocompromised nonobese diabetic (NOD) severe combined immunodeficiency (scid) γ mice bearing Raji cell xenografts. In NSG mice bearing Raji tumors co‐implanted with human PBMCs, single‐dose odronextamab (0.04, 0.1, 0.4, and 1.0 mg/kg) was administered intraperitoneally on day 0 when average tumor volume reached 220 mm3. Tumor volume in each treatment group was measured twice weekly and averages (mm3 ± SEM) are plotted. Grouped two‐way ANOVA (day 21) was performed using GraphPad PrismTM software. In Panel 3a, the gray dashed line represents a reference concentration of 1 µg/ml, at which a complete inhibition of tumor growth was achieved. In Panel 3b, asterisks indicate statistically significant differences in average tumor volume on day 21 compared with vehicle control group (***p ≤ 0.001; ****p ≤ 0.0001). ANOVA, analysis of variance; LLOQ, lower limit of quantification; NSG, NOD‐scid IL2Rgammanull; PBMCs, peripheral blood mononuclear cells; SEM, standard error of the mean
