FIGURE 1.
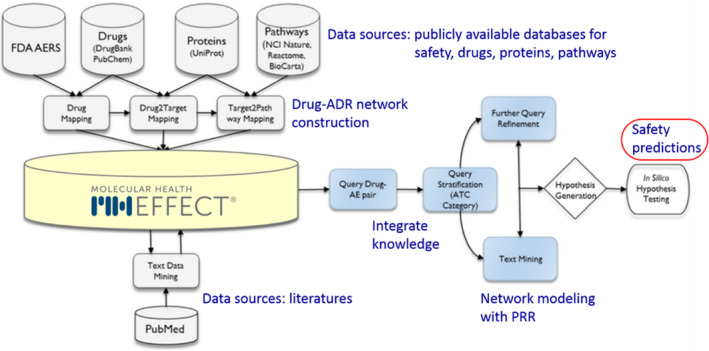
Schematic overview of the data integration process mapping FDA Adverse Event Reporting System (FAERS) data with molecular data using Molecular Health Effect. This figure was adapted from Schotland et al. 42 AE, adverse event; ATC, Anatomic Therapeutic Chemical; FDA, US Food and Drug Administration; NCI, National Cancer Institute; PRR, proportional reporting ratio
