FIGURE 2.
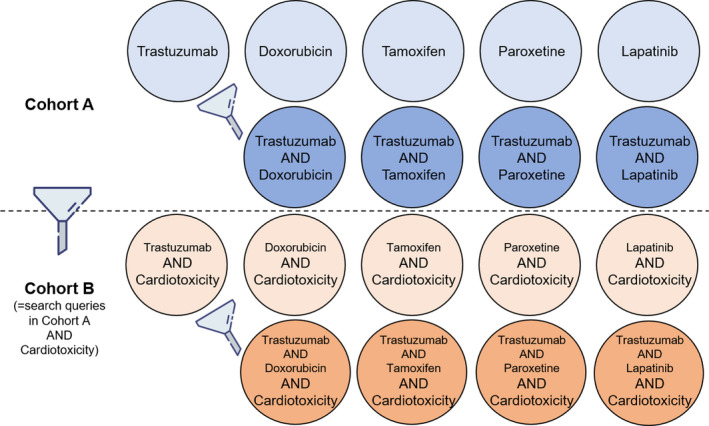
Cohort building. In cohort A, there are nine cohorts collected using drug name(s) in search queries. We collected adverse drug reaction (ADR) cases associated with each of the five investigated drugs (i.e., trastuzumab, doxorubicin, tamoxifen, paroxetine, and lapatinib). In the ADR cases associated with trastuzumab, we made four subsets of the cases reported with each of the other four drugs together. In cohort B, both drug name(s) and cardiotoxicity were used in search queries to filter out cases that do not include cardiotoxicity among reported ADRs from each of the nine cohorts. Data were collected from Q1/2008 to Q4/2019 in all cohorts
