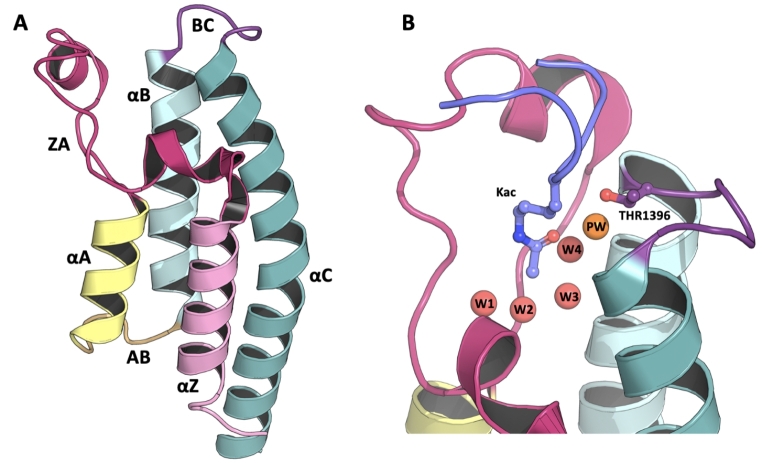
Fig. 1.
Molecular structure of a typical bromodomain fold and PHIP2 binding site. The left panel (A) shows the α-helical bundle fold (PDB-ID: 5RJI). The αZ, αA, αB and αC indicate the Z, A, B and C alpha helices, in pink, yellow, cyan and teal, respectively. ZA, AB and BC show the connecting loops of same name, in fuchsia, orange and purple, respectively. The right panel (B) shows the PHIP2 binding site in complex with H4K5acK8ac-like peptide (PDB-ID: 7BBP). The conserved bromodomain 4 water-network is showed in red spheres with the additional PHIP2 water in orange. The atypical threonine 1396 and acetylated lysine (Kac) are displayed in purple and lilac sticks, respectively