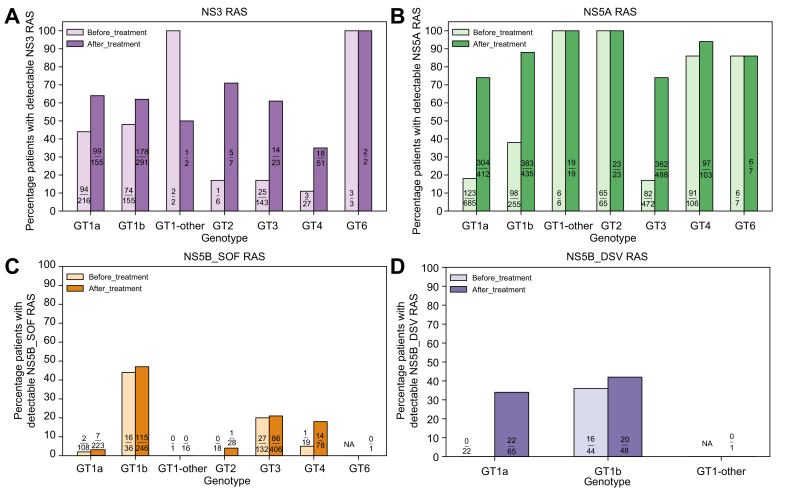
Fig. 1.
Prevalence of resistance-associated substitutions in DAA-naïve and -experienced patients with HCV.
HCV sequences from virologic failures treated with combination regimens containing a PI (SIM, GZR, PAR/r, GLE, ASV, VOX, BOC, TVR), an NS5AI (LDV, DCV, EBR, VEL, OMB, PIB), or an NI (SOF)/NNI (DSV) were evaluated for the prevalence of RASs in NS3, NS5A, NS5B_SOF, and NS5B_DSV, respectively. HCV DAA-naïve patients were included to estimate the natural RAS prevalence. Patients who harbored ≥1 RAS variant listed in the 2020 EASL recommendations on treatment of hepatitis C were counted. ASV, asunaprevir; BOC, boceprevir; DAA, direct-acting antiviral; DCV, daclatasvir; DSV, dasabuvir; EBR, elbasvir; GLE, glecaprevir; GT, genotype; GT1-other, any GT1 subtypes except GT1a and GT1b; GZR, grazoprevir; LDV, ledipasvir; NA, not available; NI, nucleoside inhibitor; NNI, non-nucleoside inhibitor; NS5AI, NS5A inhibitor; OMB, ombitasvir; PAR/r, paritaprevir/ritonavir; PI, protease inhibitor; PIB, pibrentasvir; RAS(s), resistance-associated substitution(s); SIM, simeprevir; SOF, sofosbuvir; TVR, telaprevir; VEL, velpatasvir; VOX, voxilaprevir.