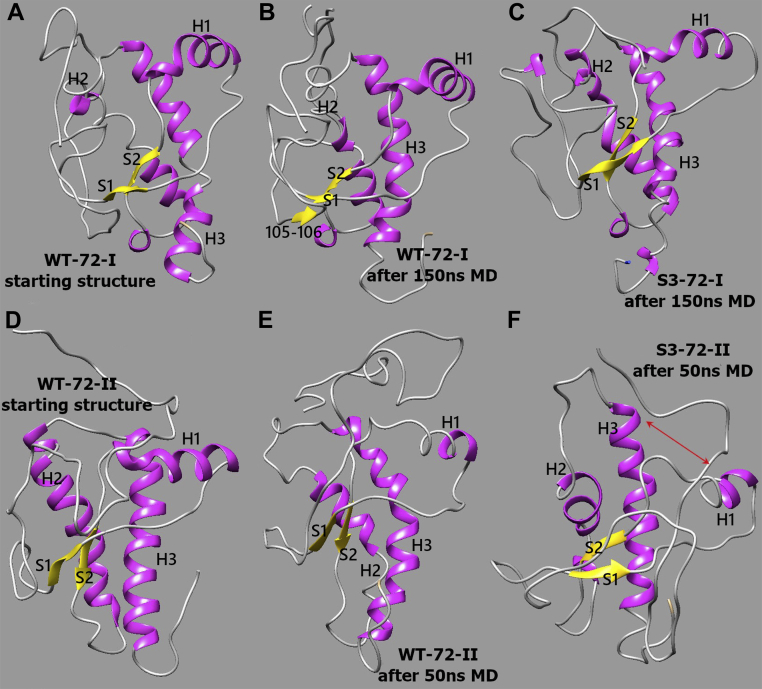
Figure 4.
Comparison of mPrP 72–231 structures. Comparison of WT-72-II and S3-72-II mPrP 72–231 structures. A, original WT structure based on 2L39.pdb that was used to build WT-72-I and S3-72-I models. B, WT-72-I representative conformation after 150 ns MD simulations: α-helix H2 partially unfolded; β-sheet S1–S2 reduced to shorter β-strands; transient short β-strands appeared in the N terminus. C, S3-72-I representative conformation after 150 ns MD simulations: C-terminal regions of α-helices H2 and H3 substantially disrupted. D, original WT structure based on 2PRP.pdb that was used to build both WT-72-II and S3-72-II models. E, WT-72-II representative conformation after 50 ns MD simulations: C termini of helices partially unfolded. F, S3-72-II representative conformation after 50 ns MD simulations: region S1–H1–S2 and bundle H2–H3 shifted away from each other exposing the hydrophobic core between them; helix H1 considerably shifted toward C-terminal part of H3. MD, molecular dynamics; mPrP, mouse PrP.