Figure 6.
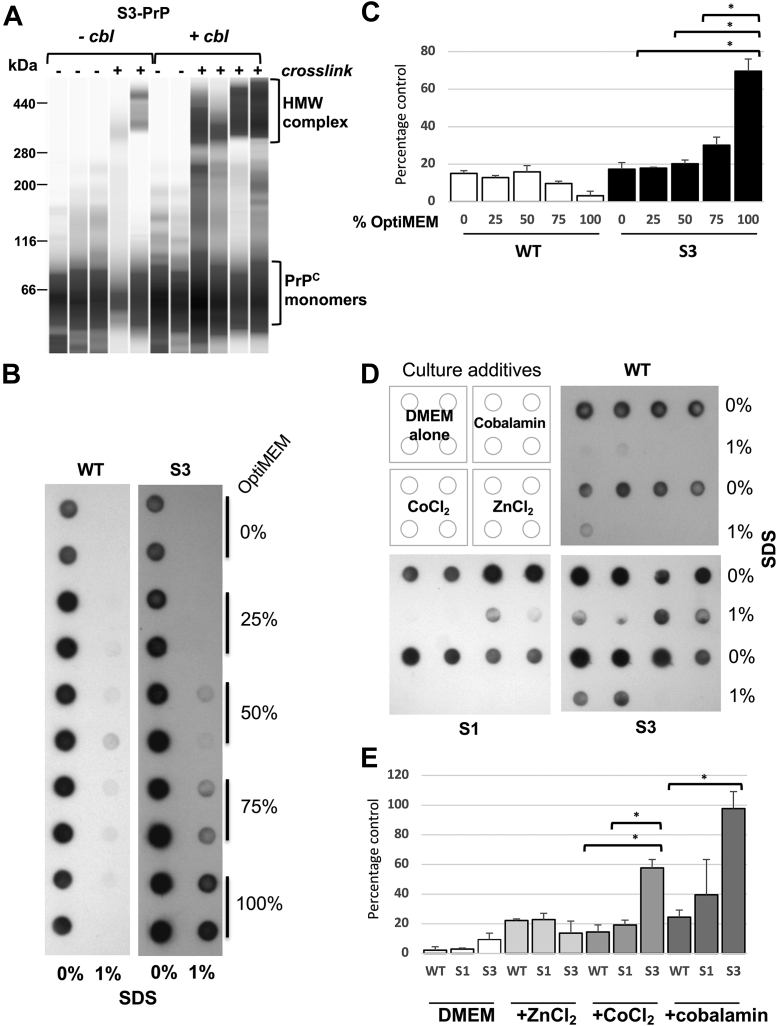
Effect of Cbl on PrP assemblies and detergent insolubility.A, cells expressing S3-PrP were grown in DMEM with or without Cbl. Cells were left untreated (−) or crosslinked in situ with 2.5% formaldehyde for 15 min at room temperature. Immunoblot detection by capillary-based Western analysis of PrP was performed from crude cellular extracts using Sha31 antibody. Diglycosylated monomeric forms of PrP with a mass of ∼35 kDa run with an apparent size of 66 kDa on this capillary-based Western analysis system (as shown previously (109)) (lower bracket). In the presence of Cbl, there was increased representation of formation of high–molecular weight (HMW) assemblies (higher bracket) with an apparent mobility of 300 to 400 kDa. B, WT-PrP and S3-PrP cells were grown for 1 week in DMEM containing increasing concentrations of Opti-MEM. Cell lysates of WT-PrP, S1-PrP, and S3-PrP generated with RIPA buffer were treated or not treated with 1% SDS (final concentration). Products were analyzed by filter-trap assay. The proteins retained by the cellulose acetate were detected by incubation with Sha31 antibody. C, quantification of A. D, WT, S1, and S3 cells were grown for 1 week in DMEM containing various additives: 0.85 μM Cbl, 0.85 μM CoCl2, and 1.12 μM ZnCl2. Cell lysates were processed as in B. E, quantification of data presented in D. Cbl, cobalamin; DMEM, Dulbecco's modified Eagle's medium; PrP, prion protein; RIPA, radioimmunoprecipitation assay buffer.