Abstract
Background
Chronic total occlusion percutaneous coronary interventions (CTO-PCI) can be highly complex and are associated with an increased risk of complications. Coronary perforation (CP) is one of the most feared complications of CTO-PCI. Awareness of the potential consequence, as well as meticulous attention to patient monitoring, can aid in rapid treatment if it happens. We present a unique case covering myocardial contrast echocardiography (MCE) characterization of interventricular septal hematoma secondary to CP associated with decompression of the hematoma into the left ventricle cavity and a favorable clinical outcome.
Case Description
This is a case of a patient with no space-occupying effect in interventricular septum before CTO-PCI showed severe chest pain after PCI. Bedside echocardiography showed thickening of the interventricular septum with the anechoic area, and contrast-enhanced echocardiography suggested the presence of interventricular septal hematoma and coronary-ventricular fistula. It was considered that retrograde CTO-PCI led to CP, which developed into an interventricular septal hematoma. The hematoma obstructed the right ventricular outflow tract (RVOT) to a lesser amount; at the same time, the perforated coronary artery created a fistula with the left ventricle, resulting in perfusion damage and myocardial ischemia to some extent, although the patient’s vital signs remained stable. Therefore, conservative treatment was carried out under close observation. The patient stayed stable. The hematoma was absorbed 7 days after the operation, and completely absorbed 1 month later.
Conclusions
Although most cases of myocardial hematoma caused by CP can be treated conservatively without causing acute hemodynamic damage, a myocardial hematoma can progress at any time. Closely monitoring the changes in patients’ symptoms and vital signs; mastering the location of the perforated coronary artery, the size of the hematoma and the hemodynamic abnormalities can help clinicians quickly make further treatment plans. Echocardiography coupled with contrast-enhanced ultrasonography, which is non-invasive, safe, cost-effective, and bedside-operable may accurately indicate the location, size of the hematoma, whether there is a shunt, as well as observe the hemodynamic changes and myocardial perfusion in real-time.
Keywords: Case report, chronic coronary total occlusion, intramyocardial hematoma, coronary perforation (CP), percutaneous coronary intervention (PCI)
Introduction
Interventricular septal hematoma is a rare complication of retrograde chronic total occlusion percutaneous coronary interventions (CTO-PCI) with a variable course (1-3). Pathophysiologically, an initial bleed may appear “contained” in surrounding tissue accompanied by the risk of progress in a relentless fashion, dissecting its way through the myocardium and, in the process, avulsing perforating vessels, which in turn bleed further, establishing a self-propagating process. The bleeding that occurs in the ventricular septum would lead to a hematoma. Here, a case of interventricular septal hematoma after right coronary artery (RCA) CTO-PCI using a retrograde approach has been reported (4). The development of ST-segment elevation, chest pain, and right ventricular outflow tract (RVOT) obstruction complicated the case. And, with the use of myocardial contrast echocardiography (MCE), we treated the patient conservatively, resulting in hematoma resolution and a satisfactory outcome. To the best of our knowledge, no case reports or series analyzing and discussing MCE findings related to the Coronary perforation (CP) as the complication of CTO-PCI within the literature (5-10). We present the following article in accordance with the CARE reporting checklist (available at https://cdt.amegroups.com/article/view/10.21037/cdt-21-707/rc).
Case presentation
A 33-year-old male patient felt chest tightness and pain across the anterior chest accompanied by radiation in the left upper limb after exercise, which could be relieved by resting for several minutes. Eight months prior, he underwent coronary artery bypass grafting surgery. Correspondingly, the ascending aorta-radial artery graft-diagonal branch, ascending aorta-saphenous vein graft-posterior descending branch, and ascending aorta-saphenous vein graft-diagonal branch was performed. The operation was smooth and chest pain was relieved postoperatively. Eight months later, symptoms reappeared. A coronary computed tomography angiogram was performed to evaluate the occlusion of the ascending aorta bypass graft to the RCA. And RCA was occluded from the proximal lumen. Besides, there was a myocardial bridge in the middle segment of the anterior descending branch. He was referred to department of cardiology in Guangdong Cardiovascular Institute of Guangdong Provincial People’s Hospital for percutaneous coronary intervention was not feasible to engage with the antegrade guiding catheter at the RCA inlet in this case. As a result, the antegrade approach could not be performed, and we chose to adopt the retrograde approach for revascularization. The septal collateral branches were evaluated by preoperative angiography, which was nontortuous and measured as 0.3–0.5 mm. Type-CC2 septal collateral consisting of continuous, small side branch-like size of the collateral according to Werner classification was selected. A Launcher 7 Fr Brite-tip XB 3.5 (Cordis, Miami, FL, USA) was engaged in the left coronary artery (LCA) and a Sion black guidewire (Asahi Intecc, Nagoya, Japan) was selected into the septal branch. Following that, a 150 cm Corsair microcatheter (Asahi Intecc) with selective contrasting performed utilizing its tip was chosen. The septal branch was successfully passed with the Sion black guidewire. Selective contrasting was performed in a retrograde manner to confirm the guidewire had entered into the distal end of RCA. The microcatheter was then followed. The intraplaque crossing was attempted and successfully achieved in the distal by changing the guidewire into Pilot 200 (Abbott Vascular, Santa Clara, CA, USA). However, the proximal advancement was failed. Reverse controlled antegrade and retrograde tracking was promptly planned. After inflating a balloon over the antegrade wire, the retrograde Pilot 200 guidewire was advanced into the space created by the balloon. The guidewire and microcatheter were advanced into the antegrade guide, the retrograde guidewire is removed, and RG3 (Asahi Intecc) was advanced until it exits from the Y connector of the antegrade guide catheter. After successful lesion crossing and balloon expanding, the RCA was stented with 2.5×33, 3.0×36, and 3.5×36 mm drug-eluting stents from distal to proximal, respectively. TIMI 3 flow was achieved after the procedure.
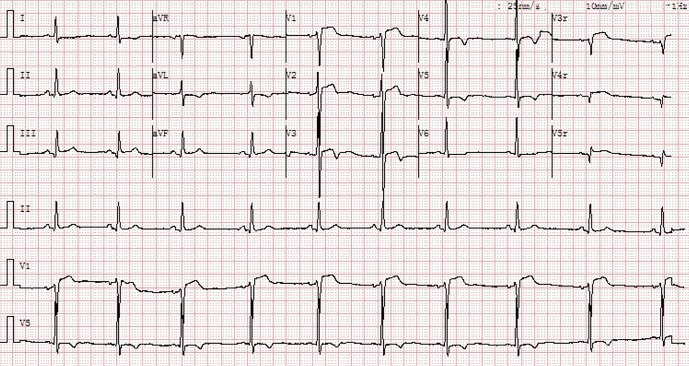
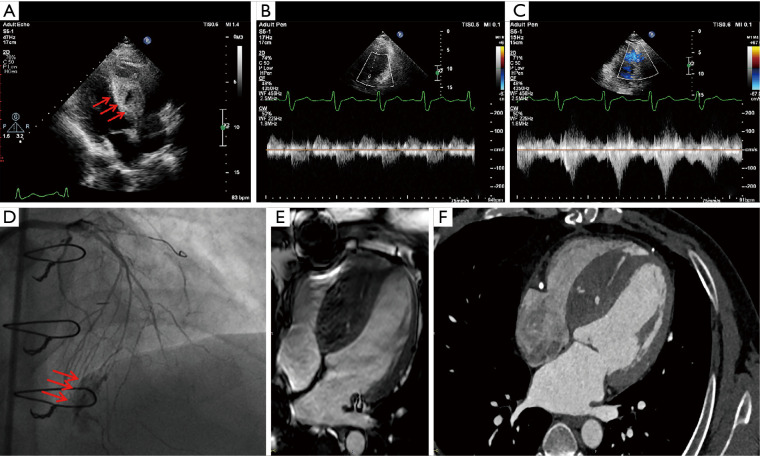
After his PCI, he developed severe chest pain and an electrocardiogram revealed anterior ST-segment elevation (Figure 1). Stent thrombosis was suspected. Bedside echocardiography measured the septal 2.0×4.0 cm in four-chamber view with 2 anechoic areas, and the larger one measured 1.8×1.6 cm. There was the obliteration of the RVOT from the hematoma with normal left ventricular function (Figure 2A-2C and Videos 1,2). A minor pericardial effusion was discovered. Repeat angiography revealed the presence of patent RCA stents. There was coronary perforation (CP) type III of the septal branch according to Ellis classification, in which contrast spilled directly into the left ventricle, and a large septal hematoma formed in the ventricle septum (Figure 2D, Video 3). Analysis of the symptom in association with the positive imaging findings suggested a clinical diagnosis of Ellis type IIIb coronary artery perforation at the collateral branch. Cardiac magnetic resonance imaging (MRI) and cardiac computerized tomography (CT) showed thickening of the ventricular septum with active bleeding, which formed ventricular septal hematoma (Figure 2E,2F). Contrast echocardiography (CE) was performed. The anechoic intra-mural cavities were asynchronously enhanced compared with peripheral tissue and communicated, which corresponded to the intramyocardial hematoma. Contrast filling of the hematoma was immediately second to the left ventricle cavity; a breach could be tracked between the hematoma and the cardiac cavity, while there was no communication between the hematoma and right ventricle or pericardial cavity (Videos 4,5).
Figure 1.
Cardiac telemetry monitor strip and an electrocardiogram showing anterior ST-segment elevation after RCA CTO-PCI using the retrograde approach, which was complicated by perforation of the first septal artery branch. RCA, right coronary artery; CTO-PCI, chronic total occlusion percutaneous coronary intervention.
Figure 2.
Imaging of ventricle septal hematoma. Post procedure echocardiography demonstrated increased interventricular septum thickness and echo-free spaces within the septum (1.8×1.6 cm) (arrows) (A). The signal of blood flow at LVOT was normal (B) and blood flow of RVOT was slightly accelerated (C). Angiogram of LAD, reveals Ellis type IIIb septal collateral perforation (arrows) into the septum and left ventricle (D). Cardiac CT and cardiac MRI showed thickening of the ventricular septum, a small amount of active bleeding, and ventricular septal hematoma (E,F). LVOT, left ventricular outflow tract; RVOT, right ventricular outflow tract; LAD, left anterior descending artery; CT, computerized tomography; MRI, magnetic resonance imaging.
Video 1.
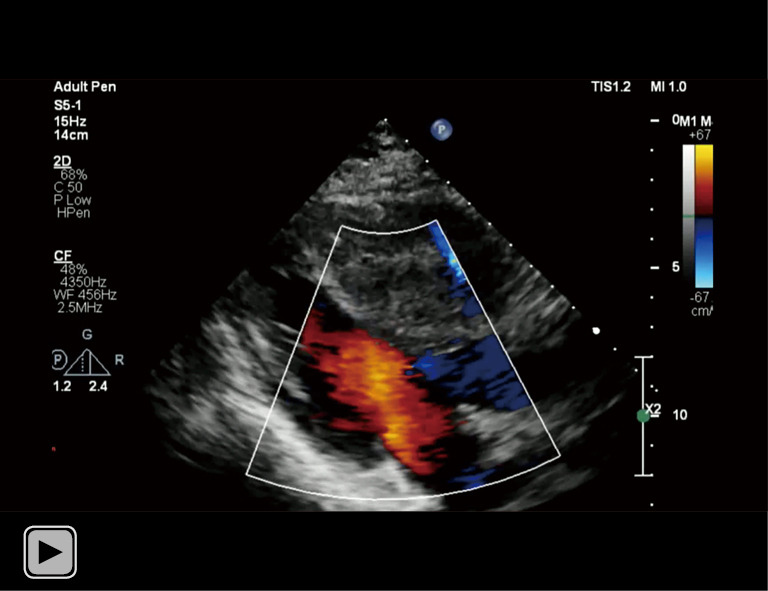
Bedside echocardiography of left ventricle outflow tract found thickening of interventricular septum with heterogeneous echogenicity.
Video 2.
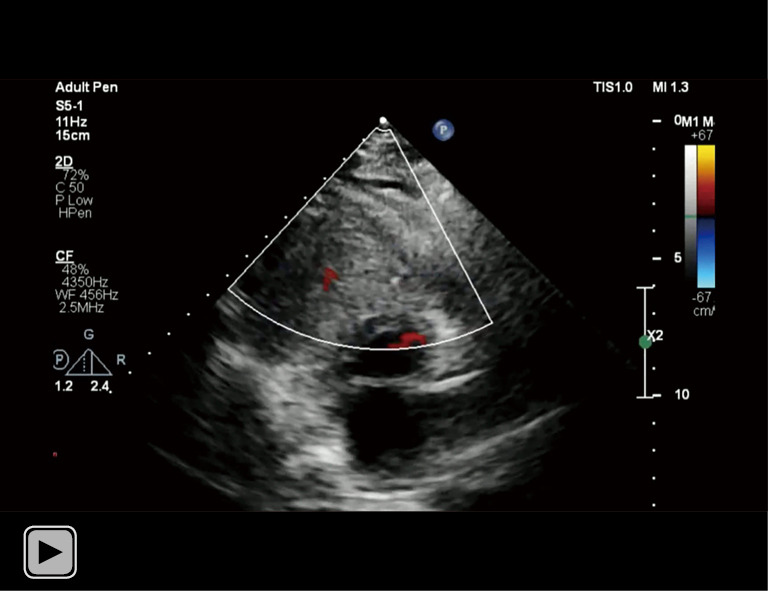
There was obliteration of the right ventricle outflow tract from the hematoma.
Video 3.
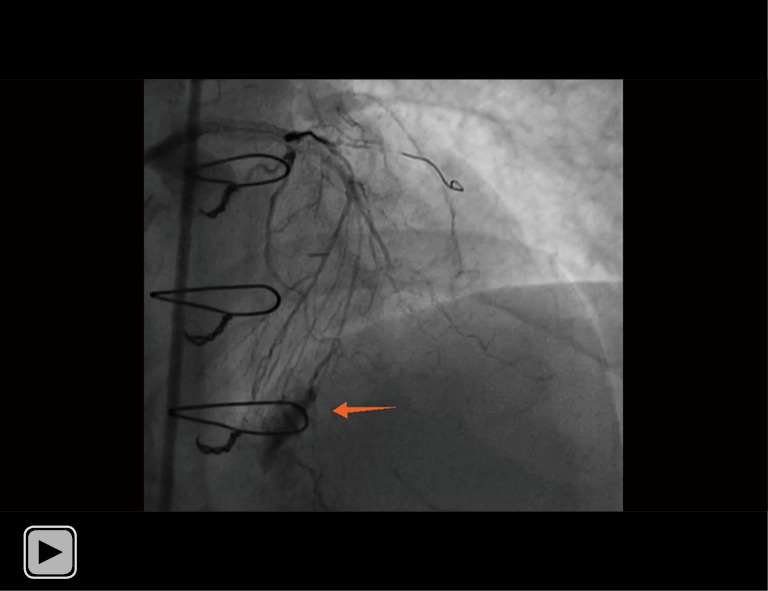
Repeated angiography revealed there was Ellis type III CP of the septal branch. The contrast spilled directly into the left ventricle, and a large septal hematoma formed in the ventricle septum, which was marked by the arrow. CP, coronary perforation.
Video 4.
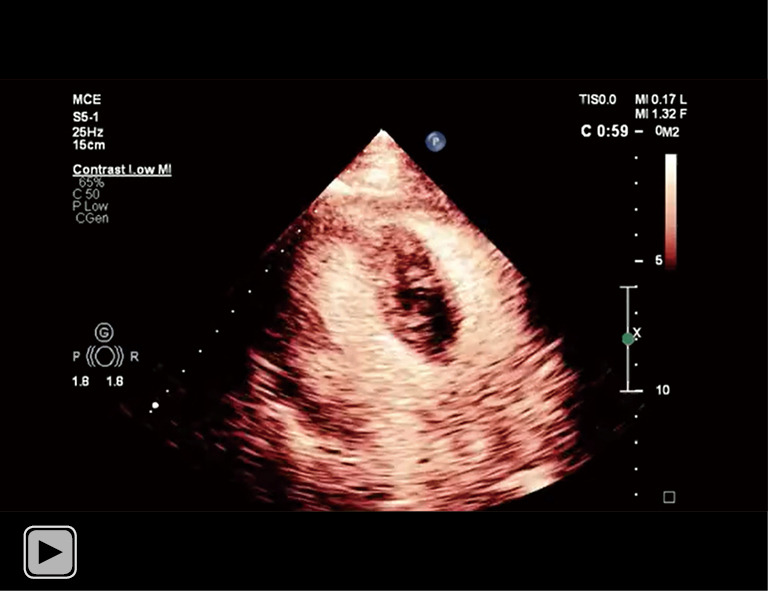
The intramyocardial hematoma were enhanced asynchronously compared with peripheral tissue. Contrast filling of the hematoma was immediately second to the cardiac chamber. A communication between the hematoma and left ventricle cavity was highlighted.
Video 5.
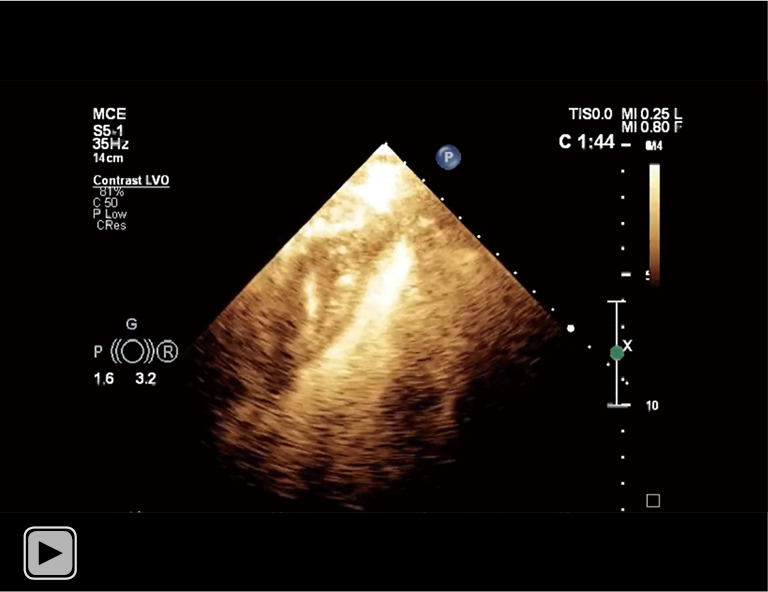
The communication between the hematoma and left ventricle cavity was much clearer under the setting of left ventricular opacification.
Considering the hematoma extravasated into the left ventricle, and the patient was hemodynamically stable, conservative management was delivered with intensive monitoring. The patient was then transferred to the cardiac care unit and treated with beta-blockers and analgesics. A repeated CE 7 days later showed no progress of the hematoma. One of the hematomas was absorbed and another larger one was decreased to 1.7×0.6 cm. RVOT’s flow was still turbulent and somewhat accelerated. We continued the conservative treatment since the patient remained stable. The hematoma showed no apparent change on serial echocardiography. The patient was asymptomatic and hemodynamically stable and therefore was discharged on hospital day 10 (Table 1). One month after PCI he was clinically stable and angina-free. A subsequent CE showed resolution of the interventricular septal hematoma and preserving of RVOT (Video 6).
Table 1. Disease progression and treatment response.
| Timeline | Patient’s condition |
|---|---|
| Initial procedure | Successful CTO-PCI was performed but with septal hematoma formation, which was treated conservatively |
| 1 h post-procedure | Echocardiogram with obstruction of the right ventricle |
| Day 10 post-procedure | Discharged from the hospital |
| 2 weeks after discharge | Seen in follow-up clinic with no anginal symptoms or limitations |
| One month after the procedure | MCE with complete resolution of a septal hematoma |
CTO-PCI, chronic total occlusion percutaneous coronary intervention; MCE, myocardial contrast echocardiography.
Video 6.
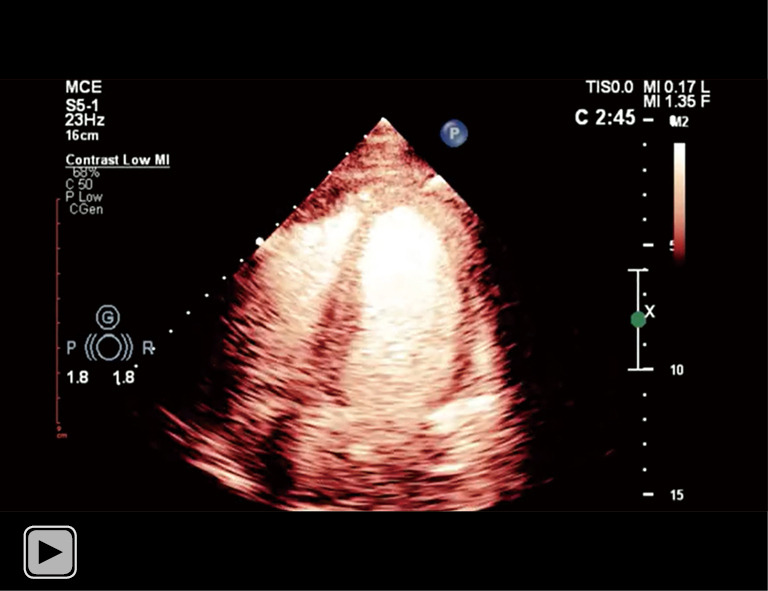
Subsequent CE during follow-up showed resolution of the interventricular septal hematoma. CE, contrast echocardiography.
All procedures performed in the study were in accordance with the ethical standards of the institutional and/or national research committee(s) and with the Helsinki Declaration (as revised in 2013). Written informed consent was obtained from the patient for publication of this case report and accompanying images. A copy of the written consent is available for review by the editorial office of this journal.
Discussion
A CTO lesion can be approached in both antegrade and retrograde manner (11). The retrograde access has significantly increased the procedural success rates in CTO-PCI. The distal cap of a CTO can be accessed through epicardial collaterals or septal branches. Perforation of collaterals during a retrograde CTO-PCI is a feared complication, which is potentially fatal (12). Septal collateral rupture usually results in a septal hematoma. Conservative echocardiography combined with MCE is the first effective examination method for suspected myocardial hematoma. It is noninvasive, safe, and economical, and it may be used near the bed. MCE can accurately display the location of hematoma breach, hematoma size, and shunt, and observe the hemodynamic changes, myocardial perfusion in real-time. The main point of this case report is the viability of using echocardiography to monitor ventricular hematoma changes in septal branch perforation during CTO-PCI through retrograde route. In this example, we may take lessons from the experience of diagnosis, treatment, and follow-up for comparable patients by reviewing the diagnosis and treatment.
The retrograde approach refers to CTO access of the distal cap via collateral vessels. Angiographic collaterals can be divided into septal and epicardial channels (11,12). When the instrument moves through the blocked coronary artery, it is simple to damage the arterial wall and produce CP, causing the artery wall’s integrity to be compromised and the coronary blood to extravasate to the surrounding tissue. The perforation type was determined based on the Ellis classification: I, extraluminal crater without extravasation; II, pericardial or myocardial blushing without contrast jet extravasation; IIIa, extravasation through frank (>1 mm) perforation; IIIb, “cavity spilling”, which refers to perforations with contrast spilling directly into either the left ventricle, coronary sinus or another anatomic circulatory chamber. Patients who suffered type I or II CP showed a tendency of good prognoses, while those with type III perforations, in which locate at epicardial collaterals, had a higher mortality rate. As epicardial collaterals are fragile and associated with high mortality, where CP communicates with the pericardial cavity, which, if left untreated, may quickly lead to pericardial tamponade and hemodynamic failure (13). To inhibit forward blood flow, coil embolization or balloon dilatation are frequently used; if there is persistent contrast media extravasation following balloon dilatation, membrane-coated stents should be investigated (14,15). An emergency pericardiocentesis is required when there is cardiac tamponade. Although right ventricular hematomas are often big, the majority of cases may be treated conservatively without causing acute hemodynamic abnormalities. Conservative treatment includes close observation of vital signs and/or fluid resuscitation to improve right ventricular preload. Dobutamine can be considered for the treatment of intractable hypotension, especially when hematoma causes RVOT obstruction. Left ventricular free wall hematoma tends to be benign and can also be treated conservatively (16,17). In rare cases, the hematoma can be drained to the low-pressure right ventricle through the spontaneously formed ventricular septal defect, or the hematoma can be drained by fenestration of a small balloon via a guidewire, which is a better method than using coils to block the supply vessels on both sides of the hematoma (1). Although drainage of hematoma can prevent the expansion of hematoma, the absorption of hematoma may take several weeks, and until then the hemodynamic abnormalities caused by hematoma will not be relieved (18). Data analysis from 1,000 patients registered OPEN-CTO found that 89 of enrolled patients developed CP after the intervention, of which 43 (48%) needed clinical treatment. Notably, 21% with perforation who received clinical treatment died, whereas none died in those who did not require treatment. Proximal vascular involvement, epicardial collateral origin, and large perforation were identified as features associated with adverse outcomes (19). In our case, delayed Ellis type IIIb coronary artery perforation at the collateral branch might be caused by mechanical stress. Delayed perforation can result in a catastrophic event after the implantation of coronary stents. Fortunately, the perforation led to a hematoma that extravasated into the left ventricle. The patient was hemodynamically stable and required no special treatment. Conservative management was delivered with intensive monitoring.
Because of the adverse consequences of CP via the epicardial collateral pathway, some scholars have tried to adopt an interventricular septal collateral pathway for CTO-PCI. Even if CP occurs, the comparatively closed myocardial tissue can limit the extent of leakage. Perforation can, however, occur on dissection lines between ventricular spiral muscles. And if the blood dissects its way through the myocardium while avulsing perforating vessels, which will, in turn, establish a self-propagating process to become septal hematomas. Cardiovascular MRI is a useful noninvasive technique for determining the presence of intramyocardial hemorrhage. Since T1 and T2 relaxation techniques are sensitive to blood products, they frequently assist in diagnosis. The elevated myocardial densities of paramagnetic hemoglobin degradation products (deoxyhemoglobin, methemoglobin) and blood degradation products (ferritin and hemosiderin) will exploit the T2 and T2* shortening effects, which makes intramyocardial hemorrhage present as a hypointense signal within a region of hyperintensity (20). Subacute (1–4 weeks) hematoma will show heterogenous signal intensity, with areas of high signal owing to liquidation. After the hematoma is organized, there will be increased heterogeneity within the hematoma with a general decrease in average T1 but a general increase in average T2 values. Hematomas will uniformly fail to enhance the administration of gadolinium-based contrast agents, owing to their avascular nature (21). Although cardiac MRI is a highly accurate imaging modality, there are several practical limitations to the use of the technique. When compared to echocardiography, cardiac MRI examinations are time-consuming and there is a high demand for patients’ compliance and heart rhythm. For patients with implanted pacemakers and cardiac devices, there are problems with scanning. The echocardiography can show the size and location of the myocardial hematoma, the break in the hematoma, and whether there is a shunt. The acoustic characteristics of hematoma sandwiched between two layers of the myocardium can be seen as anechoic in acute bleed or cystic-like, an echo-lucent cavity. The outer wall is made of the myocardium and pericardium while the inner wall, which faces the ventricular cavity, includes the remaining part of the myocardium and endocardium. It can change over time and show three different components, one is the original myocardium, the second is the anechoic area caused by active coronary-ventricular septal fistula, and the third will be the thrombosis gradually formed in the anechoic area. The pressure in the anechoic area of the ventricular septum will rise as active bleeding increases; after the pressure in the coronary artery and the anechoic area of the ventricular septum has been progressively balanced, the coronary-ventricular septal fistula may be blocked. And the ventricular septal hematoma could be gradually absorbed. The differential diagnosis includes ventricular trabeculations, intracavitary thrombi, and pseudoaneurysm (22-24). Preoperative and postoperative cardiac image comparisons are crucial to rule out the likelihood of trabeculations or thrombi. Meanwhile, careful examination of the endocardial and muscular layers is extremely important. MCE has more diagnostic value than conventional echocardiography by delineating the boundary of endocardium and highlighting the continuity of myocardial echogenicity (25-29). If there is evidence of additional hematoma expansion, pericardial effusion, tamponade, or significant shunt development, more aggressive management with hematoma should be adopted.
MCE presented interventricular septal hematoma as a sparse microbubble echo in the anechoic area after several cardiac cycles, and there may be a filling defect in the myocardium near this area. When there is a breach between the hematoma and the cardiac cavity, the hematoma’s filling intensity increases and the development order is second only to the cardiac cavity where the breach is located. Moreover, the contrast agent can help to display the breaks that are difficult to observe by two-dimensional ultrasound. Contrast material is useful to define the endocardium and distinguish prominent ventricular trabeculations, thrombi. It can also highlight the communication between the ventricular cavity and endocardial or pericardial cavities to identify the pseudoaneurysm or pericardial effusion. Furthermore, previous reports of intramyocardial hematoma described the varying magnitude of enhancement on contrast transthoracic echocardiography (1,30,31). It is believed that this varying degree of enhancement is caused by active bleeding and hematoma evolution. Hence, the degree of enhancement in the hematoma can inform the clinic whether or not to continue bleeding.
The principal limitation of this case report is that it was based on retrospective observation of a single patient. We made reasonable inferences according to literature and pathophysiology, however systematic analysis and summary the role of MCE in such patients based on larger studies are still needed.
Conclusions
Septal hematomas causing hemodynamic instability are rare complications of CTO-PCI and can be life-threatening. This is a case report of isolated RV compression that was handled conservatively under MCE supervision. MCE assessment of the hematoma is a promising surveillance technique.
Acknowledgments
The authors are very grateful to the patient’s parents, who contributed the images of this article. The authors also thank Shaun Judge from CureEdit (http://wscureedit.com) for editing the language of this manuscript.
Funding: This study was supported by the General Project of Guangdong Natural Science Foundation (2021A1515012232), the Guangzhou Science and Technology Plan [201904010448], and the Clinical Transformation Research of the Guangdong Provincial People’s Hospital (2017zh04).
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. All procedures performed in the study were in accordance with the ethical standards of the institutional and/or national research committee(s) and with the Helsinki Declaration (as revised in 2013). Written informed consent was obtained from the patient for publication of this case report and accompanying images. A copy of the written consent is available for review by the editorial office of this journal.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
Footnotes
Reporting Checklist: The authors have completed the CARE reporting checklist. Available at https://cdt.amegroups.com/article/view/10.21037/cdt-21-707/rc
Peer Review File: Available at https://cdt.amegroups.com/article/view/10.21037/cdt-21-707/prf
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://cdt.amegroups.com/article/view/10.21037/cdt-21-707/coif). HF reports that this study was supported by the General Project of Guangdong Natural Science Foundation (2021A1515012232), the Guangzhou Science and Technology Plan [201904010448], and the Clinical Transformation Research of the Guangdong Provincial People’s Hospital (2017zh04), and the payments were paid to HF personally. The other authors have no conflicts of interest to declare.
References
- 1.Fairley SL, Donnelly PM, Hanratty CG, et al. Images in cardiovascular medicine. Interventricular septal hematoma and ventricular septal defect after retrograde intervention for a chronic total occlusion of a left anterior descending coronary artery. Circulation 2010;122:e518-21. 10.1161/CIRCULATIONAHA.110.976555 [DOI] [PubMed] [Google Scholar]
- 2.Araki M, Murai T, Kanaji Y, et al. Interventricular Septal Hematoma after Retrograde Intervention for a Chronic Total Occlusion of a Right Coronary Artery: Echocardiographic and Magnetic Resonance Imaging-Diagnosis and Follow-Up. Case Rep Med 2016;2016:8514068. 10.1155/2016/8514068 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Frisoli TM, Afana M, Mawri S, et al. Respect the Septal Perforator: Septal Artery Perforation During CTO PCI Resulting in Massive Interventricular Septal Hematoma and Biventricular Cardiac Obstructive Shock. JACC Cardiovasc Interv 2017;10:e91-2. 10.1016/j.jcin.2017.02.018 [DOI] [PubMed] [Google Scholar]
- 4.Rathore S, Katoh O, Tuschikane E, et al. A novel modification of the retrograde approach for the recanalization of chronic total occlusion of the coronary arteries intravascular ultrasound-guided reverse controlled antegrade and retrograde tracking. JACC Cardiovasc Interv 2010;3:155-64. 10.1016/j.jcin.2009.10.030 [DOI] [PubMed] [Google Scholar]
- 5.Akam-Venkata J, Lemler M, Pirolli T, et al. Evolution of Interventricular Septal Hematoma: Echocardiographic Diagnosis. CASE (Phila) 2020;5:39-42. 10.1016/j.case.2020.10.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Araki M, Murai T, Kanaji Y, et al. Interventricular Septal Hematoma after Retrograde Intervention for a Chronic Total Occlusion of a Right Coronary Artery: Echocardiographic and Magnetic Resonance Imaging-Diagnosis and Follow-Up. Case Rep Med 2016;2016:8514068. 10.1155/2016/8514068 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Abdel-Karim AR, Vo M, Main ML, et al. Interventricular Septal Hematoma and Coronary-Ventricular Fistula: A Complication of Retrograde Chronic Total Occlusion Intervention. Case Rep Cardiol 2016;2016:8750603. 10.1155/2016/8750603 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Padalino MA, Speggiorin S, Pittarello D, et al. Unexpected interventricular septal hematoma after ventricular septal defect closure: intraoperative echocardiographic early detection. Eur J Echocardiogr 2007;8:395-7. 10.1016/j.euje.2006.06.001 [DOI] [PubMed] [Google Scholar]
- 9.Yoneyama F, Matsubara M, Sakamoto H, et al. Interventricular septal hematoma associated with congenital heart surgery: A case report and literature review. J Thorac Cardiovasc Surg 2017;153:e55-7. 10.1016/j.jtcvs.2016.10.053 [DOI] [PubMed] [Google Scholar]
- 10.Gu X, He Y, Luan S, et al. Dissection of the interventricular septum: Echocardiographic features. Medicine (Baltimore) 2017;96:e6191. 10.1097/MD.0000000000006191 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Touma G, Ramsay D, Weaver J. Chronic total occlusions - Current techniques and future directions. Int J Cardiol Heart Vasc 2015;7:28-39. 10.1016/j.ijcha.2015.02.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dash D. Complications encountered in coronary chronic total occlusion intervention: Prevention and bailout. Indian Heart J 2016;68:737-46. 10.1016/j.ihj.2016.03.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Boukhris M, Tomasello SD, Azzarelli S, et al. Coronary perforation with tamponade successfully managed by retrograde and antegrade coil embolization. J Saudi Heart Assoc 2015;27:216-21. 10.1016/j.jsha.2015.02.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tarar MN, Christakopoulos GE, Brilakis ES. Successful management of a distal vessel perforation through a single 8-French guide catheter: Combining balloon inflation for bleeding control with coil embolization. Catheter Cardiovasc Interv 2015;86:412-6. 10.1002/ccd.25939 [DOI] [PubMed] [Google Scholar]
- 15.Ben-Gal Y, Weisz G, Collins MB, et al. Dual catheter technique for the treatment of severe coronary artery perforations. Catheter Cardiovasc Interv 2010;75:708-12. 10.1002/ccd.22331 [DOI] [PubMed] [Google Scholar]
- 16.de Vos AM, van der Schaaf RJ. Subepicardial haematoma, a rare and potentially lethal complication of CTO-PCI: case of an exceptional recovery after conservative management. BMJ Case Rep 2014;2014:bcr2014204767. 10.1136/bcr-2014-204767 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kajander OA, Leskelä P, Virtanen MPO. Complete recovery of the right ventricle from a large intramyocardial hematoma complicating percutaneous coronary intervention. J Cardiol Cases 2016;13:175-7. 10.1016/j.jccase.2016.01.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Danek BA, Karatasakis A, Tajti P, et al. Incidence, Treatment, and Outcomes of Coronary Perforation During Chronic Total Occlusion Percutaneous Coronary Intervention. Am J Cardiol 2017;120:1285-92. 10.1016/j.amjcard.2017.07.010 [DOI] [PubMed] [Google Scholar]
- 19.Gasparini GL, Merella P, Mazzarotto P, et al. Retrograde approach-related epicardial collateral channel perforation successfully treated with simultaneous bilateral coils embolization: A case illustration and review. Cardiovasc Revasc Med 2018;19:879-86. 10.1016/j.carrev.2018.03.004 [DOI] [PubMed] [Google Scholar]
- 20.Kali A, Tang RL, Kumar A, et al. Detection of acute reperfusion myocardial hemorrhage with cardiac MR imaging: T2 versus T2. Radiology 2013;269:387-95. 10.1148/radiol.13122397 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Watson WD, Ferreira VM, Sayeed R, et al. Serial Cardiac Magnetic Resonance of an Evolving Subacute Pericardial Hematoma. Circ Cardiovasc Imaging 2019;12:e009753. 10.1161/CIRCIMAGING.119.009753 [DOI] [PubMed] [Google Scholar]
- 22.Megaly M, Xenogiannis I, Abi Rafeh N, et al. Retrograde Approach to Chronic Total Occlusion Percutaneous Coronary Intervention. Circ Cardiovasc Interv 2020;13:e008900. 10.1161/CIRCINTERVENTIONS.119.008900 [DOI] [PubMed] [Google Scholar]
- 23.Khandaker MH, Espinosa RE, Nishimura RA, et al. Pericardial disease: diagnosis and management. Mayo Clin Proc 2010;85:572-93. 10.4065/mcp.2010.0046 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wu K, Huang Z, Zhong Z, et al. Predictors, treatment, and long-term outcomes of coronary perforation during retrograde percutaneous coronary intervention via epicardial collaterals for recanalization of chronic coronary total occlusion. Catheter Cardiovasc Interv 2019;93:800-9. 10.1002/ccd.28093 [DOI] [PubMed] [Google Scholar]
- 25.Botrous C, Bioh G, Patel A, et al. Contrast echocardiography facilitates appropriate management of hospitalized patients with coronavirus disease 2019 (COVID-19) and suspected right ventricular masses: case series. Eur Heart J Case Rep 2021;5:ytaa575. [DOI] [PMC free article] [PubMed]
- 26.Weinsaft JW, Kim RJ, Ross M, et al. Contrast-enhanced anatomic imaging as compared to contrast-enhanced tissue characterization for detection of left ventricular thrombus. JACC Cardiovasc Imaging 2009;2:969-79. 10.1016/j.jcmg.2009.03.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zhang Y, Li Y, Yang Y, et al. Usefulness of contrast echocardiography in the diagnosis of left ventricular pseudoaneurysm. QJM 2020;113:741-2. 10.1093/qjmed/hcaa105 [DOI] [PubMed] [Google Scholar]
- 28.Gollol-Raju N, Olearczyk B, Johnson R, et al. Pseudo-pseudoaneurysm: a rare and unexplored mechanical complication of myocardial infarction. J Am Soc Echocardiogr 2007;20:1317.e1-3. 10.1016/j.echo.2007.03.005 [DOI] [PubMed] [Google Scholar]
- 29.Ishida T, Yasu T, Arao K, et al. Images in cardiovascular medicine. Bedside diagnosis of cardiac rupture by contrast echocardiography. Circulation 2005;112:e354-5. 10.1161/CIRCULATIONAHA.105.538348 [DOI] [PubMed] [Google Scholar]
- 30.Ezad S, Wardill T, Talwar S. Intramyocardial Haematoma Complicating Chronic Total Occlusion Percutaneous Coronary Intervention: Case Series and Review of the Literature. Cardiovasc Revasc Med 2022;34:142-7. 10.1016/j.carrev.2021.01.015 [DOI] [PubMed] [Google Scholar]
- 31.Hirai T, Nicholson WJ, Sapontis J, et al. A Detailed Analysis of Perforations During Chronic Total Occlusion Angioplasty. JACC Cardiovasc Interv 2019;12:1902-12. 10.1016/j.jcin.2019.05.024 [DOI] [PubMed] [Google Scholar]