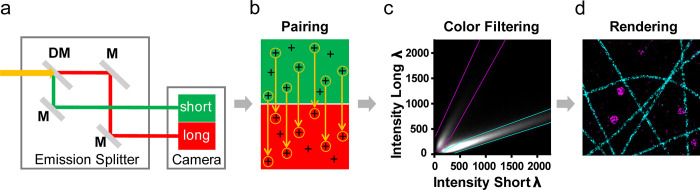
Figure 1.
Spectral demixing (SD) DNA-PAINT principle and workflow. (a) Schematic beam path of the emission splitter installed between the microscope body (not displayed) and camera system (indicated). All dyes (here, dual-color example with ATTO-655/700) are excited by a single laser line (e.g., 647 nm), and the mixed emission (yellow) is split via a dichroic mirror (DM) and 100% mirrors (M) into “short” (green) and “long” (red) wavelength channels onto a single camera. (b) The localizations detected in both channels are subjected to a custom “pair-finding” algorithm that identifies corresponding localization pairs. Random (unpaired) localizations are excluded. (c) The channel-specific intensity values (short/long) of all localization pairs are plotted into a 2D intensity histogram. Color-specific masks (cyan/magenta dotted lines) are designed to minimize color crosstalk and maximize inclusion of localizations. Each included localization is assigned to a color channel according to the masks. (d) Example of a rendered dual-color SD-DNA-PAINT image of microtubules (cyan) and clathrin-coated vesicles (magenta).