Conflict of interest
The authors have no conflict of interest to declare.
The patients in this manuscript have given written informed consent to the publication of their case detail.
Dear Editor,
We describe a subacute cutaneous lupus erythematosus (SCLE) case developed after vaccination with mRNA COVID‐19 vaccine. 1
A 30‐year‐old Italian woman presented due to the sudden occurrence of papules and plaques on her face and upper back. The eruption started following a day spent outdoors, ten days after receiving the SARS‐CoV‐2 mRNA vaccine second dose (Pfizer, Cominarty). At the time of our consultation, we observed purplish, erythematous, and scaly papules and plaques on the upper back (Fig. 1a), cheeks, temples, and forehead (Fig. 2a). The rest of the skin was unaffected.
Figure 1.
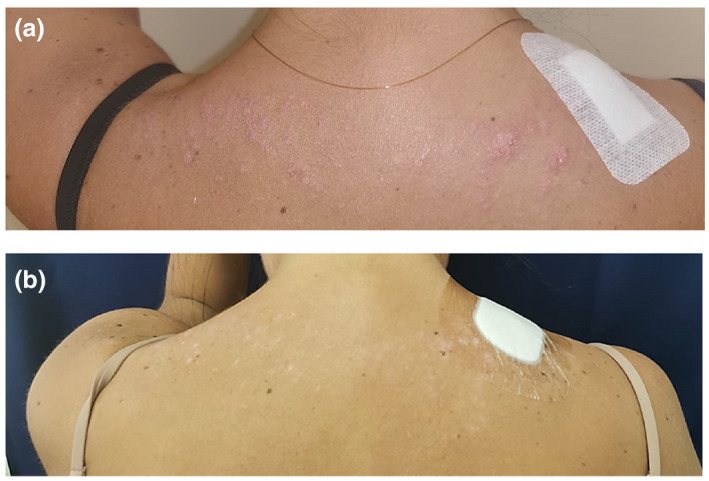
(a) Eruption on the upper trunk and erythematous plaques of the face. Note the small, erythematous, and slightly scaly papules of the back. (b) Complete resolution of the eruption, after three weeks of therapy, was achieved. Still, post‐inflammatory hypopigmented macules are visible on the back.
Figure 2.

(a) Erythematous plaque of the face (b) Complete resolution after the therapy.
She reports that she has not changed her habits, has not introduced new drugs or suffered from new diseases in the last year. However, her past medical history was complex: she was born with biliary tract atresia, operated on with Kasai surgery. She developed portal hypertension and was treated with ursodeoxycholic acid for primary biliary cholangitis. Past blood chemistry highlighted strong positivity for anti‐double strain DNA (anti‐dsDNA) and mild positivity for anti‐nucleosomes and anti‐histones antibodies. Past rheumatological ruled out signs of connective tissue disease and recommended annual follow‐up.
Skin biopsy showed hyperkeratosis, superficial perivascular and perifollicular lymphocytic infiltration and vacuolization of the basement membrane.
Blood exams showed marked leukopenia and thrombocytopenia, complement consumption, increased anti‐dsDNA, anti‐Sm, and anti‐nRNP and new positivity for anti‐Ro/SSA, compared to the last panel of exams.
The histological characteristics, positivity to anti‐Ro/SSA antibodies and clinical manifestations led to the diagnosis of SCLE. 2
Suspecting a vaccine cause, we evaluated the probability of an adverse drug reaction using the Naranjo scale. 3 We considered timing, the absence of new drugs introduced or changes in habits and the previous case reports retrieved in literature, some of which have similar cases. 4 According to the Naranjo scale, we considered the SCLE onset as probably triggered by the SARS‐CoV‐2 vaccination.
We started systemic steroid treatment with a suboptimal prednisone dose (0.4 mg/kg/day) due to the increased infectious risk and daily furoate mometasone topical cream. After three weeks, therapy led to an almost complete resolution of the skin lesions, still with residual hypopigmentation on the back (Figs 1b and 2b).
We then referred the patient to the rheumatologist to evaluate the introduction of systemic hydroxychloroquine.
Among the possible explanations, we know that tumor necrosis factor‐alpha (TNF‐α) and interferon‐gamma (IFN‐γ) are increased by infections and vaccinations, which lead to cytokine release and CD4‐type 1 helper T cell (Th1) recruitments. High levels of Th1 cells and inflammatory state have been reported in affected skin of patients with SCLE manifestations, which can explain flare episodes in predisposed patients. 5
In this case, the patient had positive antibodies specific for LE (ds‐DNA and anti‐histones) but not anti‐Ro/SSA. Until now, she had never manifested any clinical signs of LE except for an alteration of the hemogram, probably caused by splenomegaly secondary to portal hypertension.
With this case report, we aim to increase the awareness of the rare skin manifestations elicited by Sars‐Cov‐2 vaccines. We recommend dermatologists to carefully evaluate any skin reactions that may arise after a vaccination.
Data availability statement
Data available on request from the authors.
References
- 1. Kreuter A, Burmann S‐N, Burkert B, Oellig F, Michalowitz A‐L. Transition of cutaneous into systemic lupus erythematosus following adenoviral vector‐based SARS‐CoV‐2 vaccination. J Euro Acad Dermatol Venereol 2021; 35: e733–735. 10.1111/jdv.17514 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Aringer M, Costenbader K, Daikh D et al. 2019 European League Against Rheumatism/American College of Rheumatology Classification Criteria for Systemic Lupus Erythematosus. Arthritis Rheumatol 2019; 71: 1400–1412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Adverse Drug Reaction Probability Scale (Naranjo) in Drug Induced Liver Injury . LiverTox: Clinical and Research Information on Drug‐Induced Liver Injury [Internet]. Bethesda, MD: National Institute of Diabetes and Digestive and Kidney Diseases; 2012. [PubMed] [Google Scholar]
- 4. Kreuter A, Licciardi‐Fernandez MJ, Burmann S‐N, Burkert B, Oellig F, Michalowitz A‐L. Induction and exacerbation of subacute cutaneous lupus erythematosus following mRNA‐based or adenoviral vector‐based SARS‐CoV‐2 vaccination. Clin Exp Dermatol. 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Vera‐Recabarren MA, García‐Carrasco M, Ramos‐Casals M, Herrero C. Comparative analysis of subacute cutaneous lupus erythematosus and chronic cutaneous lupus erythematosus: clinical and immunological study of 270 patients. Br J Dermatol 2010; 162(1): 91–101. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data available on request from the authors.


