CONFLICT OF INTEREST
None to declare.
AUTHOR CONTRIBUTIONS
Mehmet Demirci: Conceptualization; Data curation; Formal analysis; Investigation; Methodology; Supervision; Writing – original draft; Writing – review & editing.
PEER REVIEW
The peer review history for this article is available at https://publons.com/publon/10.1111/odi.14082.
To the Editor,
The coronavirus 2019 pandemic caused by severe acute respiratory syndrome coronavirus 2 (SARS‐CoV‐2) is ongoing. Despite the 2 years of addressing infections caused by this virus, the immunopathogenesis mechanism is still not clearly understood (Bortolotti et al., 2021). Interactions between the innate immune system and oral microbiota can alter the host's balance between disease and health (Yu et al., 2019). It is known that the dominant genera in the oral microbiota and the lung microbiota are quite similar, but more diversity in the oral microbiota is found. The oral microbiota migrates more to the lungs, and cytokines associated with oral microbiota may affect the respiratory tissues (Bao et al., 2020). After searching Pubmed on October 1, 2021 using the keywords “Oral microbiota” and “COVID‐19”, 62 articles were found, but only four of them investigated the effects of COVID‐19 on the oral microbiota using next‐generation sequencing (NGS). Two‐hundred thirty‐seven healthy controls and 181 COVID‐19 patients included in these articles were analyzed at the genus level (Iebba et al., 2021; Ma et al., 2021; Ren et al., 2021; Wu et al., 2021). Regardless of age differences, three studies, except for Ren et al. (2021), reported that Neisseria genus shows a decrease in the oral microbiota of COVID‐19 patients compared with healthy controls (Iebba et al., 2021; Ma et al., 2021; Wu et al., 2021). Wu et al. (2021) reported that oral microbiota disruption after infection with SARS‐COV‐2 was attributed to Neisseria, and this decrease also was shown to cause suppression of important metabolic pathways, such as the host tricarboxylic acid cycle (TCA) (Wu et al., 2021). Figure 1 shows a comparison of some important genera in the oral microbiota that were found in COVID‐19 patients versus healthy controls. Although Ren et al. (2021) reported that the Neisseria genus was elevated in the oral microbiota of COVID‐19, they also reported white blood cell and lymphocyte counts positively correlated with Neisseria genus (Ren et al., 2021). It is known that a decrease in oral microbiota in terms of Neisseria genus in studies on influenza virus occurs (Bao et al., 2020). Recently, de Castilhos et al. reported that low abundance of Neisseria (especially N. subflava) led to an increase in the risk of mortality from COVID‐19, but they also found that N. subflava did not change with the SARS‐CoV‐2 viral load, a finding that seems to be contrary to the hypothesis (Castilhos et al., 2021). Neisseria genera are reported as the fourth most abundant bacterial genus in the oral microbiota of adults and nonpathogenic Neisseria species is thought to have a physiological role in preventing colonization of potential pathogens in oral and nasal sites and is also important in developing the T‐cell‐independent polyclonal IgM response and maintaining the immune ignorance in the acquired immune response (Dorey et al., 2019). Powell et al. studied the stimulation of macrophages with nonpathogenic Neisseria species in the oral microbiota of mice and reported that macrophages stimulated interleukin 6 (IL‐6) at low levels. On the contrary, they reported that IL‐6 stimulation was higher when they used ultraviolet (UV)‐inactivated Neisseria species (Powell et al., 2018).
FIGURE 1.
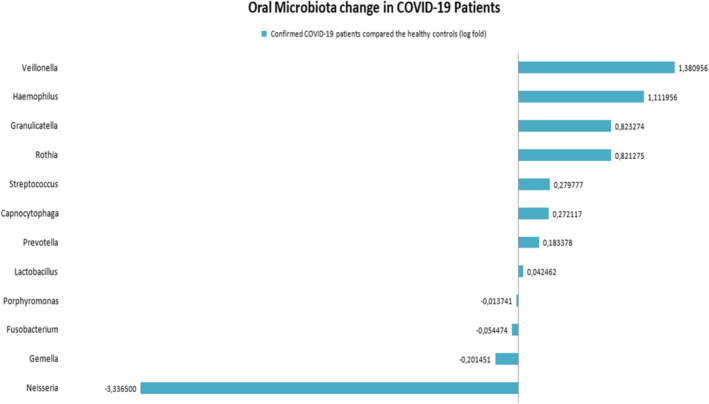
Oral Microbiota change in COVID‐19 patients (log fold)
In conclusion, the hypothesis stated that nonpathogenic Neisseria genera might be declining in the oral microbiota. Although this decrease has been reported in different studies, unfortunately, studies on the relationship between oral microbiota and COVID‐19 in this challenging period seem to be limited. Only after new standardized and comprehensive studies are performed will the effect of oral microbiota on COVID‐19 be understood.
ACKNOWLEDGMENTS
This research did not receive any specific grant from funding agencies in the public, commercial, or not‐for‐profit sectors.
Demirci, M. (2021). Could Neisseria in oral microbiota modulate the inflammatory response of COVID‐19? Oral Diseases, 00, 1–2. 10.1111/odi.14082
REFERENCES
- Bao, L. , Zhang, C. , Dong, J. , Zhao, L. , Li, Y. , & Sun, J. (2020). Oral microbiome and SARS‐CoV‐2: Beware of lung co‐infection. Frontiers in Microbiology, 11, 1840. 10.3389/fmicb.2020.01840 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bortolotti, D. , Gentili, V. , Rizzo, S. , Schiuma, G. , Beltrami, S. , Strazzabosco, G. , Fernandez, M. , Caccuri, F. , Caruso, A. , & Rizzo, R. (2021). TLR3 and TLR7 RNA sensor activation during SARS‐CoV‐2 infection. Microorganisms, 9(9), 1820. 10.3390/microorganisms9091820 [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Castilhos, J. , Zamir, E. , Hippchen, T. , Rohrbach, R. , Schmidt, S. , Hengler, S. , Schumacher, H. , Neubauer, M. , Kunz, S. , Müller‐Esch, T. , Hiergeist, A. , Gessner, A. , Khalid, D. , Gaiser, R. , Cullin, N. , Papagiannarou, S. M. , Beuthien‐Baumann, B. , Krämer, A. , Bartenschlager, R. , … Stein‐Thoeringer, C. K. (2021). Severe dysbiosis and specific Haemophilus and Neisseria signatures as hallmarks of the oropharyngeal microbiome in critically ill COVID‐19 patients. Clinical Infectious Diseases, ciab902. 10.1093/cid/ciab902. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dorey, R. B. , Theodosiou, A. A. , Read, R. C. , & Jones, C. E. (2019). The nonpathogenic commensal Neisseria: Friends and foes in infectious disease. Current Opinion in Infectious Diseases, 32(5), 490–496. 10.1097/QCO.0000000000000585 [DOI] [PubMed] [Google Scholar]
- Iebba, V. , Zanotta, N. , Campisciano, G. , Zerbato, V. , Di Bella, S. , Cason, C. , Luzzati, R. , Confalonieri, M. , Palamara, A. T. , & Comar, M. (2021). Profiling of Oral microbiota and cytokines in COVID‐19 patients. Frontiers in Microbiology, 12, 671813, 10.3389/fmicb.2021.671813 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma, S. , Zhang, F. , Zhou, F. Li, H. , Ge, W. , Gan, R. , Nie, H. , Li, B. , Wang, Y. , Wu, M. , Li, D. , Wang, D. , Wang, Z. , You, Y. , & Huang, Z. (2021). Metagenomic analysis reveals oropharyngeal microbiota alterations in patients with COVID‐19. Signal Transduction and Targeted Therapy, 6(1), 191. 10.1038/s41392-021-00614-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Powell, D. A. , Ma, M. , So, M. , & Frelinger, J. A. (2018). The commensal Neisseria musculi modulates host innate immunity to promote oral colonization. Immunohorizons, 2(9), 305–313. 10.4049/immunohorizons.1800070 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ren, Z. , Wang, H. , Cui, G. , Lu, H. , Wang, L. , Luo, H. , Chen, X. , Ren, H. , Sun, R. , Liu, W. , Liu, X. , Liu, C. , Li, A. , Wang, X. , Rao, B. , Yuan, C. , Zhang, H. , Sun, J. , Chen, X. , … Li, L. (2021). Alterations in the human oral and gut microbiomes and lipidomics in COVID‐19. Gut, 70(7), 1253–1265. 10.1136/gutjnl-2020-323826 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu, Y. , Cheng, X. , Jiang, G. , Tang, H. , Ming, S. , Tang, L. , Lu, J. , Guo, C. , Shan, H. , & Huang, X. I. (2021). Altered oral and gut microbiota and its association with SARS‐CoV‐2 viral load in COVID‐19 patients during hospitalization. NPJ Biofilms Microbiomes, 7(1), 61. 10.1038/s41522-021-00232-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu, J. C. , Khodadadi, H. , & Baban, B. (2019). Innate immunity and oral microbiome: a personalized, predictive, and preventive approach to the management of oral diseases. EPMA Journal, 10(1), 43–50. 10.1007/s13167-019-00163-4 [DOI] [PMC free article] [PubMed] [Google Scholar]


