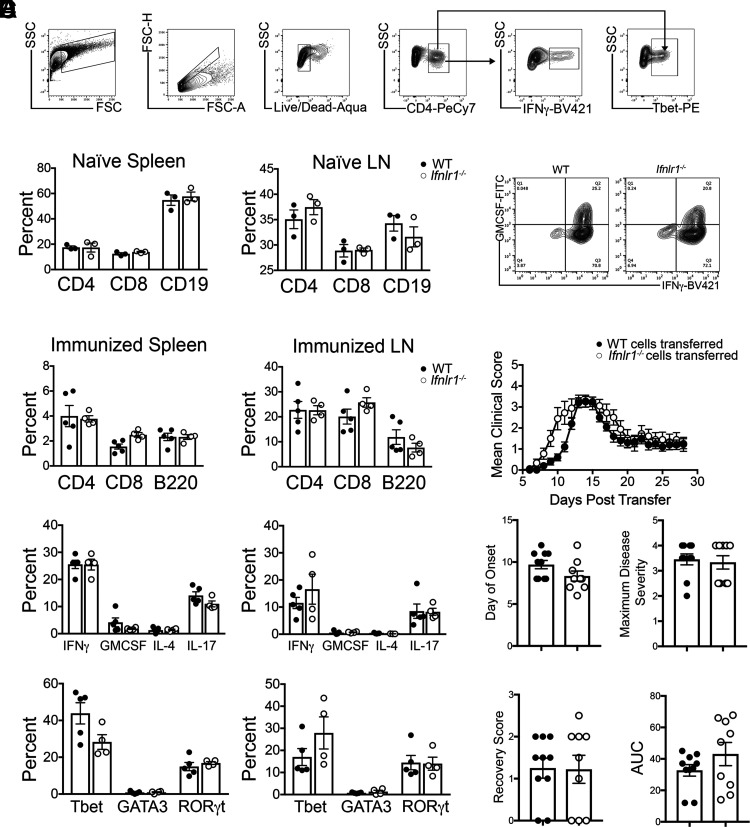
FIGURE 2.
IFNL signaling does not alter peripheral T cell priming following immunization with MOG35-55 peptide. (A) Flow cytometric gating strategy. (B) Cells were isolated from spleens and LN of naive WT and Ifnlr1−/− animals. Percentage of CD4+, CD8+, and CD19+ cells were gated and quantified from live cells. Data are representative of two independent experiments; n = 3/genotype shown. (C) WT and Ifnlr1−/− mice were actively immunized with MOG35-55 peptide. Cells were collected from spleen and draining LN 11 d following immunization. Flow cytometric analysis was performed on cells prior to stimulation to determine percentages of CD4+, CD8+, and B220+ cells. CD4+ cells were further gated on various cytokines and transcription factors; quantification is shown. Data are representative of two independent experiments; n = 5 WT and n = 4 Ifnlr1−/− animals shown. (D) Cells from (C) were stimulated and cultured in the presence of immunizing peptide and Th1-promoting cytokines with irradiated WT APCs. (D) After stimulation, MOG35-55–specific WT and Ifnlr1−/− CD4+ Th1 clones were analyzed for expression of GM-CSF and IFN-γ by flow cytometric analysis. (E) A total of 107 CD4+ Th1 clones from (D) were injected retro-orbitally into naive WT recipients. EAE clinical course was monitored. Clinical onset of EAE, maximum disease score, score at recovery, and area under the curve (AUC) was quantified for each mouse. Data are pooled from two independent experiments; n = 10 animals with WT cells transferred and n = 9 animals with Ifnlr1−/− cells transferred. Data are presented as means ± SEM.