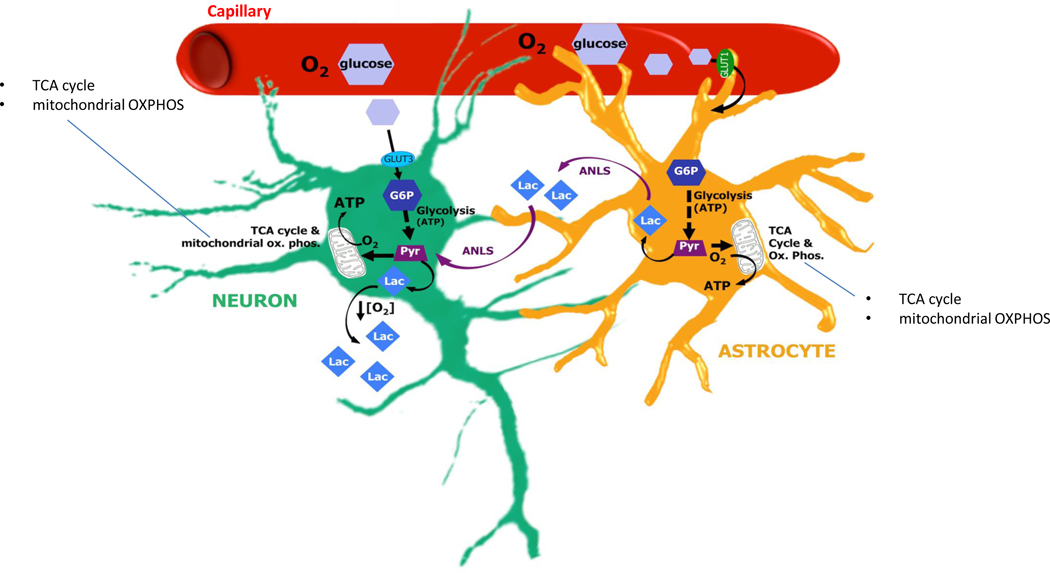
Figure 2. Bioenergetic insufficiency may underpin delirium in multiple scenarios.
Neurons and astrocytes both use glucose supplied by the microvasculature to generate ATP by glycolysis. In addition, in the astrocyte–neuron lactate shuttle (ANLS), lactate synthesised by astrocytes after glycolysis can be exported for use by neurons, which convert the lactate to pyruvate that is imported into the mitochondria and used to fuel the tricarboxylic acid (TCA) cycle. There are many ways in which the brain, or regions of the brain, may become dysfunctional due to energy insufficiency and there is support for the idea that these may contribute to precipitation of delirium. First, respiratory distress produces hypoxaemia and can cause brain hypoxia, limiting neuronal energy metabolism. That is, in hypoxic conditions, insufficient oxygen (O2) supply leads to impaired mitochondrial oxidative phosphorylation (OXPHOS) and insufficient generation of energy, in the form of ATP. In these conditions, glycolysis-generated pyruvate (Pyr), instead of being imported into the mitochondria, forms excess lactate (Lac), which can be measured in the extracellular fluid. Second, septic shock reduces blood flow, producing both hypoxia and impaired glucose supply. Third, even with adequate systemic blood flow, brain microcapillary dysfunction may produce brain tissue hypoxia and neuroglycopenia. Fourth, even with normal blood pressure, if neurovascular coupling is impaired, vessels may fail to meet the specific demands of regional neuronal activity and thereby block higher order brain functions. Fifth, systemic hypoglycaemia can lead to insufficient brain glucose supply, delirium and coma. Sixth, even with adequate delivery of glucose to the brain, insulin resistance may result in impaired glucose utilization (not shown). Seventh, altered expression of glucose transporters (GLUT1 and GLUT3), for example, in the degenerating brain, may limit glucose uptake by the endothelium, astrocytes or neurons, thereby limiting the glucose-6-phosphate (G6P) required for glycolysis and limiting the generation of Pyr required for the TCA cycle. Last,impairment of astrocyte function may limit their ability to release glycogen from intracellular stores, metabolize glucose and provide lactate to neurons for energy metabolism.