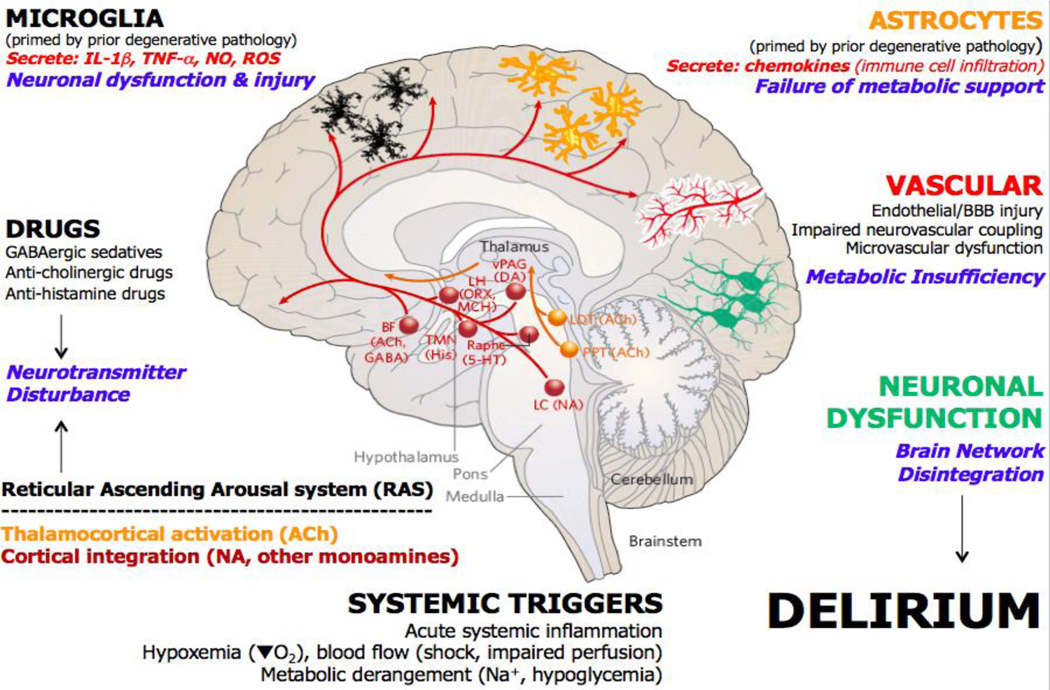
Figure 4. Major mechanisms in delirium pathophysiology.
Major perturbations leading to delirium during acute illness include robust acute systemic inflammation (involving increased circulating pro-inflammatory cytokines (such as IL-1, IL-1β and tumour necrosis factor (TNF)), pathogen-associated molecular patterns and damage-associated molecular patterns), hypoxemia, impaired blood flow and tissue perfusion, and impaired metabolism (hyponatremia, hypernatremia and hypoglycaemia). Given the altered arousal states present in different subtypes of delirium, it is widely assumed that alteration of function in the ascending arousal system may be involved. These distributed mid-brain and brainstem nuclei include strong cholinergic drive from the tegmentum to the thalamus to activate cortical arousal and multiple monoaminergic nuclei that activate the cortex to modulate and integrate cortical activation. Medications, prominently including, but not limited to, GABAergic sedatives and anaesthetics and anti-cholinergic and anti-histamine drugs, can therefore substantially alter arousal and are known to contribute to delirium. Microglia can be primed by existing pathology in the brain and further activated by acute inflammatory stimuli, secreting pro-inflammatory cytokines and reactive oxygen and nitrogen species into the surrounding brain tissue. These mediators can directly affect neuronal function but also act directly on astrocytes. Astrocytes can also be primed during chronic brain pathology, becoming hypersensitive to acute inflammatory stimulation and secreting increased levels of chemokines, which can drive recruitment of additional peripheral inflammatory cells to the brain. Activated astrocytes may also lose aspects of the energy metabolism support that they provide to neuronal function. The vasculature may become impaired, both by existing degenerative pathology and by superimposed stressors such as systemic inflammation, leading to endothelial injury and blood–brain barrier (BBB) damage, but vascular supply of oxygen and glucose may also become impaired due to microvascular dysfunction and/or impaired neurovascular coupling, contributing to a metabolic (bioenergetic) insufficiency. All of these mechanisms contribute to the most obvious proximate cause of delirium: acute neuronal dysfunction and network disintegration. 5-HT, 5-hydroxytryptamine; ACh, acetylcholine; BBB, blood–brain barrier; BF, basal forebrain; DA, dopamine; GABA, gamma-aminobutyric acid; His, histamine; LC, locus coeruleus; LDT, laterodorsal tegmental nucleus; LH, lateral hypothalamus; MCH, melanin-concentrating hormone; NA, noradrenaline; NO, nitric oxide; ORX, orexin; PPT, pedunculopontine tegmentum; ROS, reactive oxygen species; TMN, tuberomammillary nucleus; vPAG, ventral periaqueductal gray. Adapted with permission from ref.357.