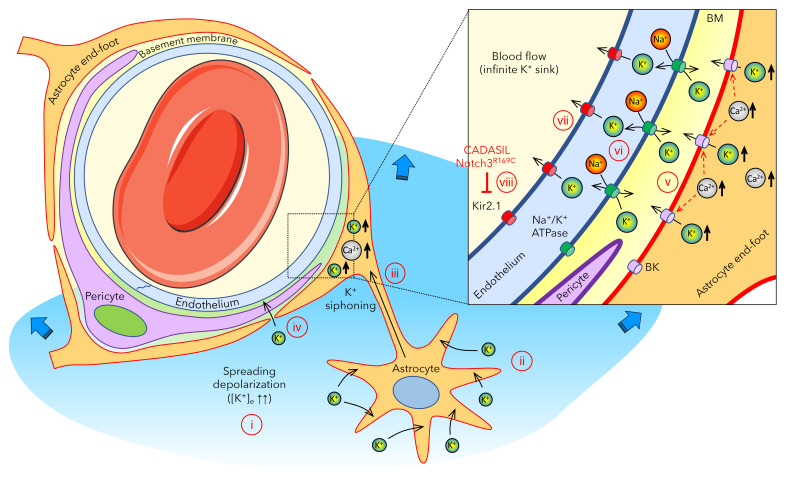
Figure 6. Proposed gliovascular mechanism of extracellular K+ regulation when local buffering mechanisms are exceeded during spreading depolarizations.
(i) Upon intense depolarization states, such as anoxic or spreading depolarization, extracellular K+ concentration ([K+]e) can rise above the 10–12 mM ceiling. (b) Astrocytes play a major role in regulating [K+]e via rapid uptake and spatial buffering through the astrocytic syncytium. (c) Astrocytes send their end feet, almost completely encasing the cerebral vasculature, including the capillary bed, providing a route for gliovascular K+ siphoning. (d) The massive rise in [K+]e during an SD might also facilitate direct entry of K+ into the perivascular space to reach the capillary endothelium. (e) Astrocyte end feet have high K+ conductance, in part, due to BK channels activated by intracellular Ca2+ elevations, such as those observed during SD, and release large amounts of K+ into the tight perivascular space. (f) This perivascular K+ is then taken up by the endothelial Na+/K+-ATPase, which is densely — and asymmetrically — localized on the abluminal membranes. (g) Endothelial cells then release the K+ into the blood stream via channels and/or pumps on the luminal membrane, including Kir2.1, which is known to be activated by elevated perivascular [K+]e. (h) Notch3R169C mutation is associated with impaired endothelial Kir2.1 channel function, linking CADASIL to impaired vascular K+ clearance.