Figure 1: ISAC Design and Characterization.
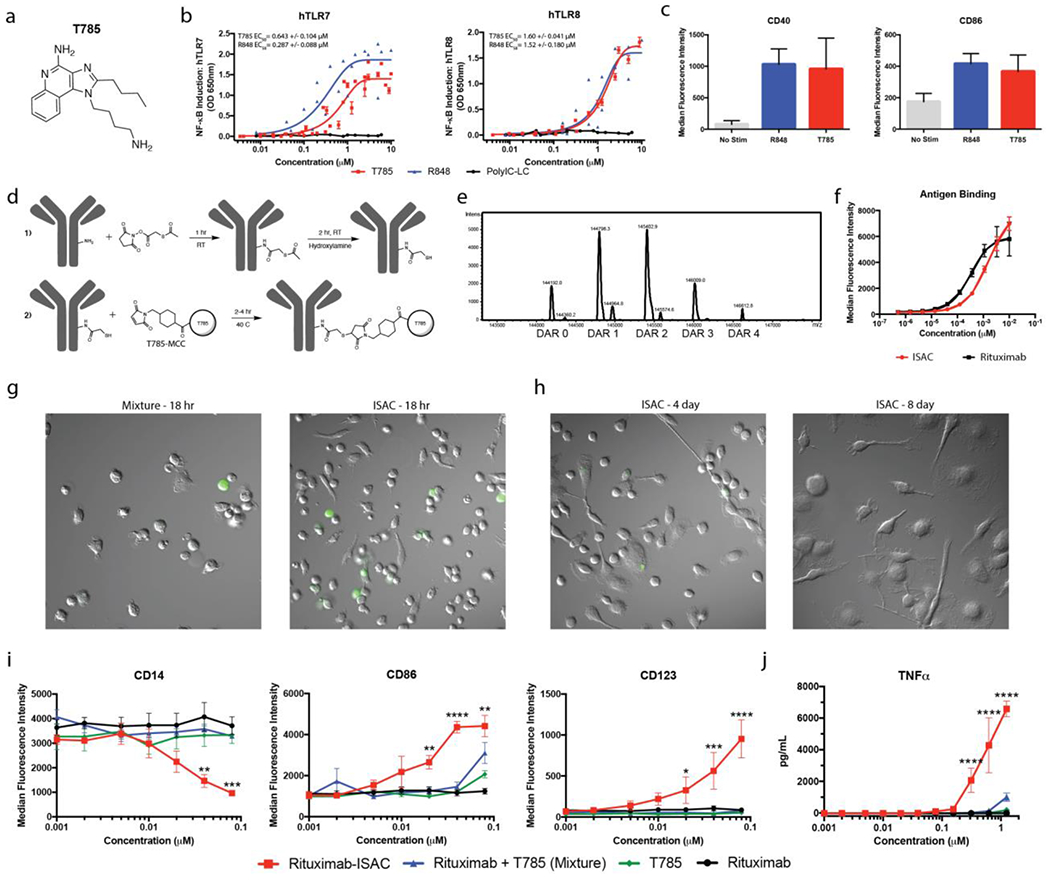
(a) Chemical structure of T785 (b) HEK-Blue-TLR7 or TLR8 reporter cells were cultured for 18 hours in the presence of T785, R848 or Poly-ICLC prior to assessment of NF-κB-induced SEAP activity. Data shown are from 8 experiments and EC50 values are calculated as mean with SEM. (c) Freshly isolated human myeloid APCs were stimulated with 1 μM of T785 or R848 for 18 hours prior to assessment of myeloid activation by flow cytometry. Data are from 1 experiment with 3 donors and are representative of >9 donors. (d) Rituximab was reacted with SATA via lysine residues to convert free amines into protected sulfhydryl groups prior to deacetylation with hydroxylamine and subsequent reaction with T785-MCC to yield the rituximab-ISAC. (e) LC-MS analysis of the rituximab-ISAC following PNGase F treatment. DAR was calculated based upon the linker-agonist mass addition of 606 g/mol. (f) Fluorescently labeled rituximab or rituximab-ISAC was incubated with CD20+ Toledo tumor cells at 4°C for 2 hours prior to analysis via flow cytometry. Data are from 1 experiment with triplicate samples and are representative of 2 experiments. (g-h) Freshly isolated human myeloid APCs were cultured with rituximab, T785, rituximab and T785 or the rituximab-ISAC in the presence of CFSE-labeled CD20+ Toledo tumor cells at a 3:1 effector to target ratio. The rituximab concentration is depicted on the X-axis with the concentration of T785 in these assays being consistent with the amount of T785 conjugated to the rituximab-ISAC. (g) Hoffman modulation contrast microscopy shown at 40X magnification after (g) 18 hours following stimulation with 80 nM of rituximab-ISAC. Data are representative of >10 donors. (h) Myeloid APCs were analyzed via flow cytometry 18 hours after stimulation. Data shown are from 3 donors and are representative of >10 donors (mean and SEM); *P<0.05, **P<0.01, ***P<0.001, ****P<0.0001.
