Figure 6: CL264-containing ISACs elicit tumor clearance in HER2 medium expressing xenografts and T cell mediated tumor clearance, immunologic memory, and epitope spreading in a syngeneic tumor model.
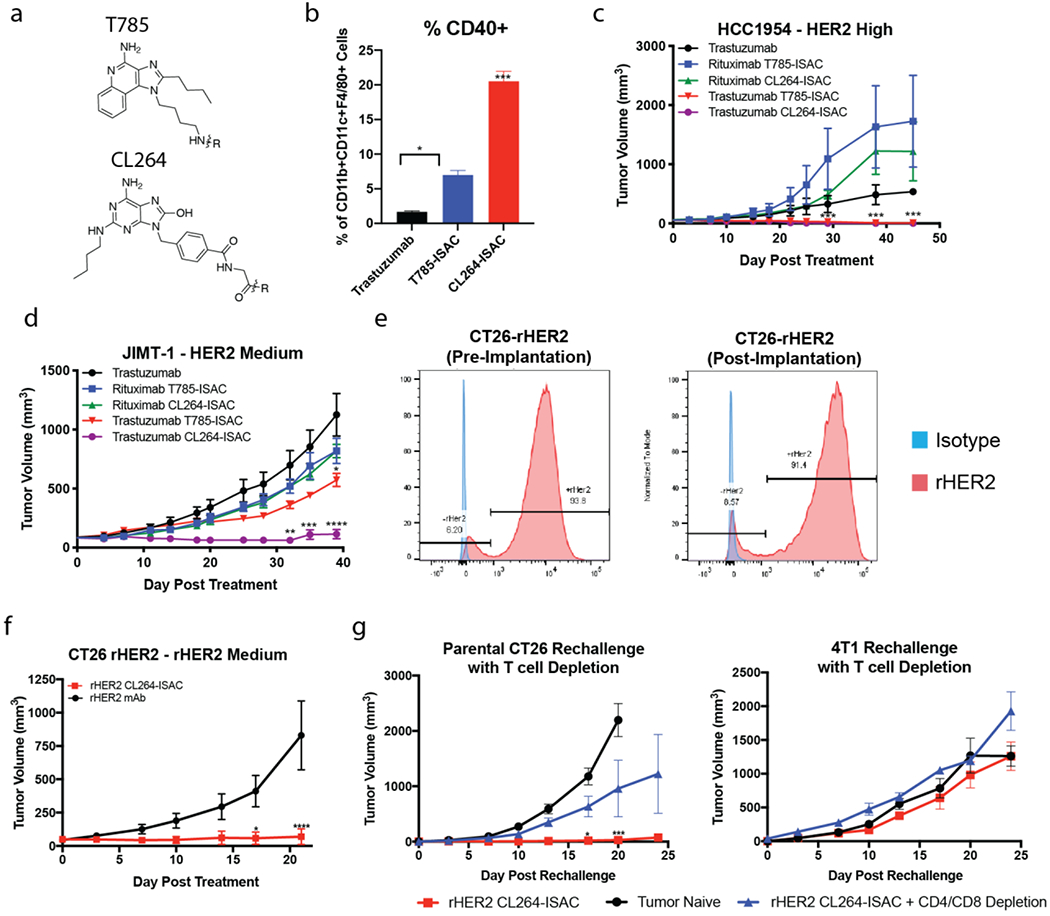
(a) Chemical structures of T785 and CL264 adjuvants used for ISAC generation. (b) NSG mice implanted with the HCC1954 tumor cell line were randomized when the tumor volume reached 50 – 75 mm3. Mice were treated with once via intraperitoneal injection of 5 mg/kg trastuzumab, trastuzumab T785-ISAC or trastuzumab CL264-ISAC. Tumors were harvested 20 hours post administration, processed to a single cell suspension, and analyzed by flow cytometry to assess activation of tumor-infiltrating myeloid APCs. (c-d) NSG or Rag2/IL2rg double knockout mice were implanted with the indicated human tumor cell line and randomized when the tumor volume reached 50 – 75 mm3 (HCC1954) or 75 – 150 mm3 (JIMT-1). Mice were treated via intraperitoneal injection with 5 mg/kg of rituximab, trastuzumab, trastuzumab T785-ISAC, trastuzumab CL264-ISAC or the respective isotype-ISACs, every 5 days for a total of 6 treatments. (e) rHER2 expression was measured on CT26-rHER2 tumor cells in culture prior to implantation (left flow plot) and in tumors nine days post-implantation (right flow plot) by flow cytometry with fluorescently-conjugated anti-rHER2 antibody (red) or an isotype control (blue). (f) Balb/c mice were implanted with the CT26-rHER2 tumor cell line and were randomized when the tumor volume reached 50 mm3. Mice were then treated via intraperitoneal injection with 10 mg/kg of mouse anti-rat HER2 or mouse anti-rat HER2 CL264-ISAC every 5 days for a total of 6 treatments. Data shown are from 1 experiment with 8 mice per arm and are representative of 3 experiments. (g) Anti-rat HER2 CL264-ISAC treated mice that experienced complete tumor regression for >21 days after their last treatment were challenged with parental CT26 (left flank) and 4T1 cell lines (right flank), with or without CD4 and CD8 T cell depletion (n =3 each). Tumor naïve mice (n = 6) challenged with the parental CT26 and 4T1 cell lines were included as controls. (a-i) Data are shown from individual experiments with 3-6 mice per arm and are representative of 1-4 experiments with a minimum of 3 mice per arm in each experiment. Data are shown as mean with SEM and statistics are shown with *P<0.05, **P<0.01, ***P<0.001, ****P<0.0001.
