Abstract
Many of our daily activities, such as riding a bike to work or reading a book in a noisy cafe, and highly skilled activities, such as a professional playing a tennis match or a violin concerto, depend upon the ability of the brain to quickly make moment-to-moment adjustments to our behavior in response to the results of our actions. Particularly, they depend upon the ability of the neocortex to integrate the information provided by the sensory organs (bottom-up information) with internally generated signals such as expectations or attentional signals (top-down information). This integration occurs in pyramidal cells (PCs) and their long apical dendrite, which branches extensively into a dendritic tuft in layer 1 (L1). The outermost layer of the neocortex, L1 is highly conserved across cortical areas and species. Importantly, L1 is the predominant input layer for top-down information, relayed by a rich, dense mesh of long-range projections that provide signals to the tuft branches of the PCs. Here, we discuss recent progress in our understanding of the composition of L1 and review evidence that L1 processing contributes to functions such as sensory perception, cross-modal integration, controlling states of consciousness, attention, and learning.
Keywords: layer 1, top-down processing, neocortex, GABAergic interneurons, pyramidal cell dendrites, predictive coding
1. INTRODUCTION
The neocortex is the largest and most complex part of the mammalian brain, comprising over 75% of the gray matter volume in humans. It is where sensory information acquired by different sensory organs is integrated with our memories to generate signals that determine our actions. The computations that take place in this brain area are essential for sensory perception, fine motor control, memory, cognition, and behavioral flexibility. The latter is likely the key to understanding why the neocortex emerged and then expanded to such a remarkable extent during mammalian evolution. After all, prior to the neocortex, brains were already effective and capable of processing sensory information, controlling movement, and learning.
Neocortical functions such as sensory perception depend upon the integration of two types of signals: information provided by the sensory organs telling the brain about the surrounding environment (bottom-up signals) and internally generated information (top-down information) that provides expectations, predictions, information on behavioral saliency, and learning rules, signals that generally provide context and enable behaviorally relevant responses at a given moment. Understanding the organization and function of the neocortex requires understanding how this integration of top-down with bottom-up information takes place.
The neocortex consists of several highly interconnected areas responsible for specific functions such as sensation (e.g., visual, somatosensory, olfactory), motor actions, and executive functions. The organization of the neocortex is hierarchical, with functionally related areas connecting to each other in specific fashions (Coogan & Burkhalter 1993, D’Souza & Burkhalter 2017, Felleman & Van Essen 1991). The connectivity is extensive. For instance, there are 305 connections described among 32 areas processing visual information and 62 pathways linking 13 cortical areas processing somatosensory/motor information in nonhuman primates in the original Felleman & Van Essen (1991) study. Understanding how different areas of the cortex interact and how the cortex communicates with the rest of the brain is key to deciphering how the cortex performs its functions. There are three main categories of intercortical connections: feedforward connections from lower to higher areas, feedback connections from higher to lower areas, and lateral connections between areas at the same level (reviewed in Callaway 2004, Gilbert & Sigman 2007, Felleman & Van Essen 1991). Each of these connections originates from and targets specific cortical layers. For example, in primary somatosensory cortex (S1), somatosensory information, the feedforward bottom-up input in this area, arrives from the thalamus in layer 4 (L4); then it is relayed to the basal dendrites of pyramidal cells (PCs) in layer 2/3 (L2/3), which project to the basal dendrites of layer 5 (L5) PCs (Douglas & Martin 1991, 2004). In contrast, feedback information (the top-down signals) targets layers other than L4 and most prominently layer 1 (L1).
Top-down and bottom-up inputs carry very distinct types of information; therefore, they need to differentially impact the activity of the PCs, the output neurons of the neocortex. All of the inputs arriving to compartments that are not electrotonically isolated summate to produce a composite response that then determines their net impact on the activity of the cell, with each input contributing proportionally to its relative effective strength. It would be very difficult for a top-down input to have a significant impact on the output of a PC (e.g., an attentional signal that is supposed to suppress or significantly enhance the activity of that PC) if it arrived at a compartment(s) also receiving bottom-up inputs. To solve this issue, PCs, the pinnacle of cortical evolution, have evolved a unique structure, with a long apical dendrite that terminates in fine dendritic branches (the dendritic tuft). These dendritic branches are electrotonically segregated from the basal dendrites and the somato-axonal area, have a complex morphology, and use specialized signaling properties to perform nonlinear operations on their inputs, thus providing the solution that enables pyramidal neurons to receive a rich diversity of top-down inputs independently from bottom up/local inputs in deeper layers.
The apical tufts of the PCs in L2/3 and L5 are housed in the outermost layer of the neocortex, L1 (Figure 1). This thin layer is a highly conserved laminar compartment present throughout all neocortical areas and mammalian species. It is thought to mediate the integration of contextual and cross-modal information in top-down signals with the input specific to a given area, enabling flexible and state-dependent processing of feedforward sensory input arriving deeper in the cortical column. To perform this critical role, L1 possesses a number of unique properties. L1 completely lacks somata of excitatory neurons; it is a cell-sparse layer containing less than 0.5% of all the cells in a cortical column (Meyer et al. 2011). In addition to the apical dendritic tufts, L1 contains a high density of horizontal fibers conveyed by massive projections from a diverse set of regions, including higher-order cortical regions, cortical areas processing different sensory modalities, callosal fibers, neuromodulatory sources, and higher-order thalamic nuclei, and from other subcortical structures. These projections fill a large fraction of L1 space and are the source of the top-down information (see Section 2.1).
Figure 1.
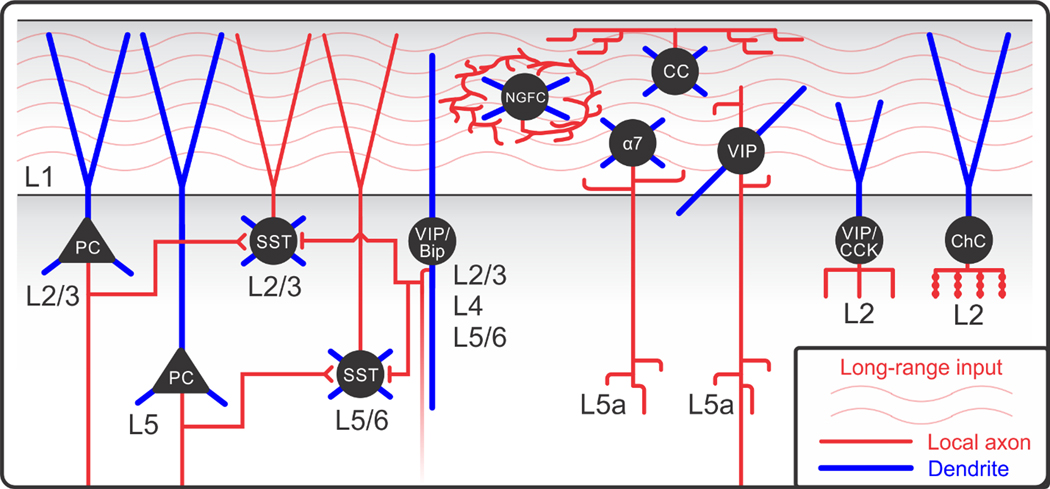
The composition of neocortical L1. L1 is the only layer lacking excitatory cell somata. It contains several structures that mediate the integration of top-down and bottom-up information in PCs, the output neurons of the cortex located in deeper layers, via synapses on their distal dendritic tufts in L1. Top-down information is conveyed to L1 by dense axonal projections, including feedback projections from cortical areas higher in the processing hierarchy, axons from higher-order thalamocortical neurons mediating cortico-thalamo-cortical loops, and axons from neurons in neuromodulatory centers. L1 also contains a rich population of GABAergic INs, including four types of INs with somata in L1: NGFCs, α7, CanCs, and VIPs. L1 also contains a significant proportion of the dendrites of at least three populations of INs with somata in L2/3, including VIP/Bips, VIP/CCK, and ChCs. Lastly, L1 contains the ascending axon of SST-expressing Martinotti cells located in L2/3 and infragranular layers. L2/3 and L5—6 SST INs receive powerful local excitatory input, but so far they do not seem to receive long-range inputs. Abbreviations: α7, α7 nicotinic receptor–expressing interneuron; CanC, canopy cell; ChC, chandelier cell; IN, interneuron; L, layer; NGFC, neurogliaform cell; PC, pyramidal cell; SST, somatostatin; VIP, vasoactive intestinal peptide–expressing interneuron; VIP/Bip, vasoactive intestinal peptide–expressing bipolar interneuron; VIP/CCK, cholecystokinin- and vasoactive intestinal peptide–expressing interneuron.
L1 also contains a remarkably diverse population of resident GABAergic interneurons (INs) as well as the dendrites of several IN subtypes in L2/3 [including vasoactive intestinal peptide (VIP)-expressing bipolar INs, VIP/cholecystokinin (CCK)-expressing INs, and chandelier cells (ChCs)] and the ascending axons of somatostatin (SST)-expressing Martinotti cells in L2/3 and infragranular layers (Figure 1). These inhibitory sources can gate the flow of information arriving to L1 with specific spatiotemporal patterns and sculpt how this information is delivered to the PCs.
It has been clear for decades that L1 is the primary target of projections relaying top-down signals. However, the lack of understanding of its cellular composition and the possible mechanisms by which inputs arriving at the distal dendritic branches of PCs could impact PC function led investigators to refer to L1 as enigmatic or, as David Hubel (1982) called it, a crowning mystery. As we document in this review, significant progress has been made in understanding both the composition of L1 and the mechanisms by which the distal dendritic branches of PCs can process and integrate distal inputs (see Section 2). Each cortical area receives multiple distinct top-down signals, and throughout the review we address the many ways in which structures in L1 have been adapted to preserve the specificity of these information streams, including highlighting evidence for L1 sublaminar organization. In Section 3 of the review, we discuss recent evidence suggesting that functions associated with top-down processing depend upon L1. The structural and functional aspects of L1 that endow it with the ability to integrate the astonishing range of top-down information streams it receives in a given neocortical area—an innovation that paved the way to our evolutionary success—are becoming clearer and more amenable to future experimentation.
2. STRUCTURAL ELEMENTS OF NEOCORTICAL LAYER 1
2.1. Corticocortical Projections to Layer 1
Communication between cortical regions is crucial to the function of the cerebral cortex and relies on both feedforward and feedback interareal projections. Feedforward projections are output connections from lower cortical areas to higher ones, while feedback projections signal from higher cortical areas to lower ones. The integration of these two streams of information likely enables increasingly complex and flexible cortical processing. Although the nature of feedback connections in the cortex is not fully understood, clear anatomical patterns have been discovered that demonstrate conserved motifs across brain regions and organisms, underscoring their functional importance.
Cortical inputs preferentially innervate specific layers in their target areas, and there is abundant anatomical evidence demonstrating common motifs in these projections (Callaway 2004). The middle layers, notably L4, the main recipient of thalamocortical (TC) sensory input in primary sensory cortices, receive in higher-order cortices feedforward input from lower-order areas. Conversely, L1 is conventionally thought to receive minor input from primary sensory thalamus (however, see Ibrahim et al. 2021) and is targeted instead by intracortical feedback inputs. Although feedback input is not strictly limited to L1, it is the most common layer for feedback input and often the most heavily innervated (Cauller 1995, D’Souza & Burkhalter 2017). L1 receives little local excitatory input (Chu et al. 2003, Jiang et al. 2015, Petreanu et al. 2009, Wozny & Williams 2011), but it contains long-range excitatory corticocortical fibers from myriad cortical origins. Among all cortical layers, L1 has the highest density of excitatory synapses (DeFelipe et al. 1999, Stepanyants et al. 2009), reflecting the abundance of tuft dendrites from PCs and the high density of long-range feedback fibers. The colocalization of these synapse-rich dendrites and axons of feedback projections suggests that L1 plays a central role in the local integration of feedback and sensory (or feedforward) information throughout the cortex.
Particularly well-studied are the visual cortices in nonhuman primates where some of the earliest evidence of corticocortical feedback was observed, including the finding that the higher-order visual cortex V2 projects back to primary visual cortex (V1) most heavily in L1 (Kuypers et al. 1965, Rockland & Pandya 1979, Tigges et al. 1973, Wong-Riley 1979). Feedback projections from higher sensory cortical areas are not limited to primary sensory areas; higher-order visual areas can relay information to each other via L1 as well. For instance, while the middle temporal visual area (V5, or MT) projects back to V1, MT also projects to V2, V3, and V4, but most heavily to L1 (Shipp & Zeki 1989, Spatz 1977; although see Maunsell & van Essen 1983). Rockland & Pandya (1979) demonstrated a feedback chain in which the inferior temporal area TE projects to V3, V4, and V5, which then project to V2, in turn projecting to V1, all predominately in L1. In the rat visual cortex, as in primates, input from V1 to V2 terminates in the middle layers, while V2 feeds back to V1 in L1 (Miller & Vogt 1984); several higher-order visual areas also project back to V1 via L1 [and often also layer 6 (L6) as they do in macaque (Coogan & Burkhalter 1993, Juavinett & Callaway 2015)]. Work in the Virginia opossum, a marsupial, revealed feedback projections from V2 to V1 in L1 as well (Benevento & Ebner 1971). Thus, in the visual cortices of diverse species, a conserved cortical motif exists in which lower-order areas target the middle layers of higher-order areas, while feedback input from higher-order to lower-order cortices predominately targets L1.
While many anatomical studies have been performed in the visual cortex, this same feedforward/feedback motif has been observed in auditory and somatosensory cortices. Corticocortical feedback input to L1 of S1 comes from secondary somatosensory cortex (S2), primary motor cortex, insular cortex, and lateral parietal areas (Cauller et al. 1998, Friedman 1983). Higher-order somatosensory cortices feedback to each other as well. For example, in the macaque, S2 projects to the retroinsular area (Ri), Ri projects to posterior parietal cortex, the insular cortex projects to S2 and Ri, and the dysgranular insular cortex projects to S2, all predominately targeting L1 (Friedman et al. 1986). As with visual and somatosensory cortices, inputs from higher-order auditory cortex have been shown to project to L1 in the primary auditory cortex (A1) (Fitzpatrick & Imig 1980, Pandya & Rosene 1993, Pandya & Sanides 1973).
Anatomical studies in macaques have also demonstrated cross-modal projections from parietal and auditory association areas to both V1 and V2 in L1 (Rockland & Ojima 2003). Moreover, there is evidence for cross-modal input directly between sensory cortices in rodents as well (Ibrahim et al. 2016, Miller & Vogt 1984). A unifying theme here is that cortical areas for each sensory modality receive multiple streams of cross-modal information that converge in L1. More broadly, cortical processing at its very earliest (in primary sensory areas), as well as in the respective higher-order and association areas, employs L1-targeted mediated feedback. The ubiquity of this motif at different stages of cortical processing across all sensory modalities suggests that it is fundamental, underscoring its importance for sensory processing in the cortex (see Section 3.2).
Prefrontal cortices (PFCs) are also highly interconnected with projections that can target multiple layers and heavily innervate L1 (Barbas & Pandya 1989, Cruikshank et al. 2012, Pandya et al. 1971, Yeterian et al. 2012). Furthermore, some frontal areas receive input from sensory areas (Mitchell & Cauller 2001, Pandya & Kuypers 1969, Scannell et al. 1995, Vogt & Pandya 1987), including projections that predominately target L1 (Mitchell & Cauller 2001, Vogt & Pandya 1987). Extensive work by the Allen Institute (http://connectivity.brain-map.org) shows that somatosensory and visual cortices often predominately target L1 in the secondary motor cortex (M2) and anterior cingulate (ACC), respectively; auditory cortex can target L1 of M2, ACC, and prelimbic PFC.
2.1.1. Sublaminar organization of corticocortical layer 1 projections.
Although L1 is a thin structure, it seems to have a sublaminar organization. Corticocortical projections have specific localizations within L1 (Cruikshank et al. 2012, Schuman et al. 2019) (Figure 2). Some projections preferentially target the upper half of L1, while others target the lower half. This is a recurring theme also observed for TC and neuromodulatory center projections (Figure 2), as well as for the axons of GABAergic neurons (see Section 2.4). This organization can help provide specificity to the effects of distinct inputs on PCs.
Figure 2.
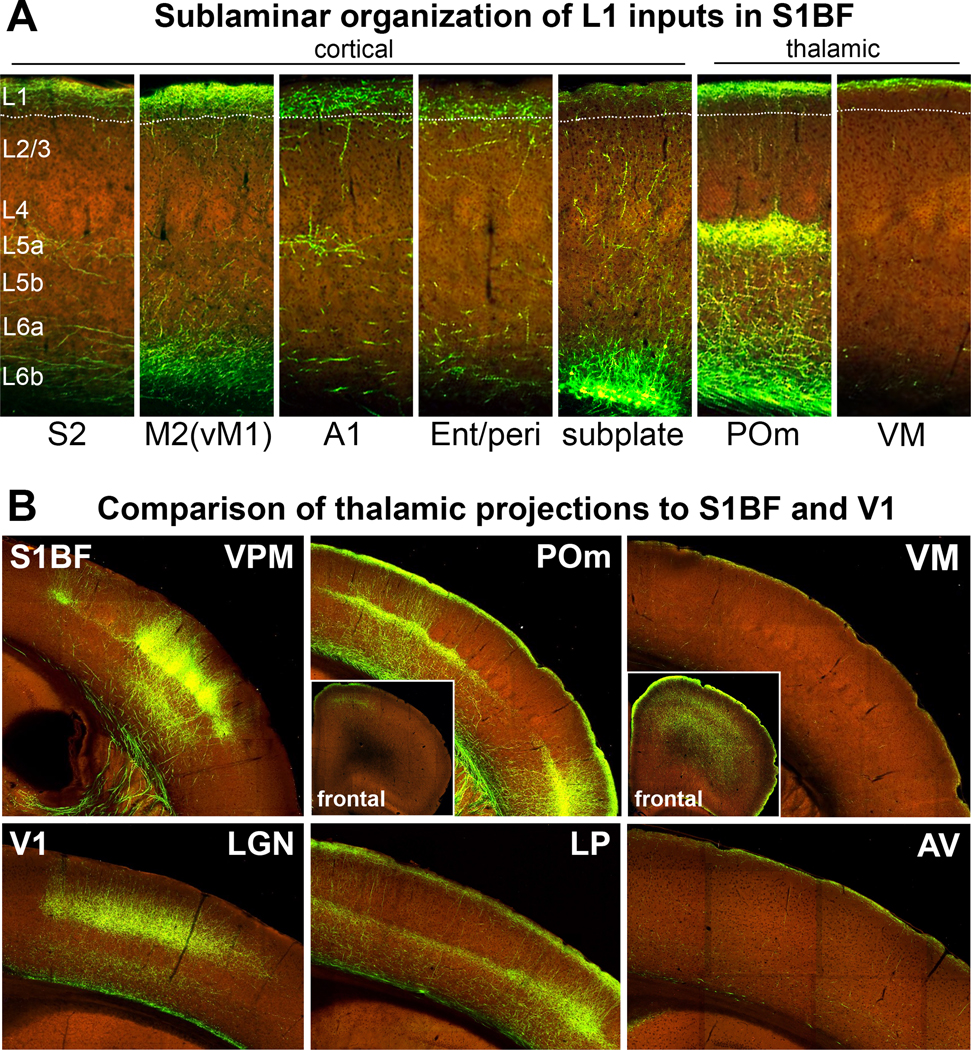
Laminar and sublaminar organization of L1 afferents in primary sensory cortex. (a) Corticocortical and thalamocortical inputs to the S1BF show specific laminar distributions and intriguing sublaminar biases within L1. Long-range cortical projections to L1 in S1BF include those from S2, M2 (also known as vM1), A1, and Ent/peri (see Section 3.5). In addition to these long-range corticocortical inputs, there is a distinct local projection to L1 from the subplate (L6b; CTGF+), with ascending collateral projections to L1 in M1/M2. Thalamocortical inputs include those from the higher-order POm and VM. These afferents exhibit specific laminar distributions and intriguing sublaminar biases within L1, with S2, vM1, and the subplate targeting superficial L1, while A1 and Ent/peri projections target mid/lower L1. The thalamocortical inputs from the POm and VM are also biased toward superficial L1. (b) Comparison of thalamocortical inputs to S1BF and V1 reveals specific trends in areal, laminar, and sublaminar distributions. Inputs from the first-order VPM and LGN relaying sensory information to S1 and V1, respectively, target L4 and L5b/6. In contrast, inputs from the higher-order POm to S1 and LP to V1 target L1 and L5a. Inputs from the higher-order VM and AV to S1 and V1, respectively, also target superficial L1 but lack the L5a projection. Moreover, their L1 projection is even more restricted in superficial L1 than the input from POm and LP. Projections from higher-order thalamic nuclei are diverse, with some exhibiting diffuse targeting of wide areas of the cortex like the VM, while others are more specific, such as the POm with projections largely restricted to somatosensory/motor areas (see insets). The laminar, sublaminar, and areal specificity of corticocortical and thalamocortical projections illustrated here likely contribute to the specific organization and processing of distinct inputs along pyramidal neuron apical dendrites. The widespread innervation of cortical areas by the VM might explain its role in global arousal (see Section 3.3). Abbreviations: A1, primary auditory cortex; AV, anteroventral thalamic nucleus; Ent/peri, entorhinal/perirhinal cortex; L, layer; LGN, lateral geniculate nucleus; LP, lateral posterior thalamic nucleus; M1, primary motor cortex; M2, secondary motor cortex; POm, posteromedial thalamic nucleus; S1, primary somatosensory cortex; S1BF, somatosensory barrel field cortex; S2, secondary somatosensory cortex; V1, primary visual cortex; VM, ventromedial thalamic nucleus; vM1, vibrissal motor cortex; VPM, ventroposteromedial thalamic nucleus. Images of fluorescently labeled projections are from the Allen Institute (http://connectivity.brain-map.org)
2.1.2. Long-range GABAergic corticocortical projections.
While most long-range corticocortical inputs to L1 are glutamatergic, there is also evidence for long-range GABAergic projection neurons in the cerebral cortex (reviewed in Melzer & Monyer 2020). Employing intersectional genetics, He et al. (2016) showed that a subpopulation of SST INs coexpressing high levels of nNos (Nos1) send axons across cortical areas, with some projections densely targeting L1. These projections could synchronize activity in multiple cortical areas.
2.2. Thalamocortical Projections to Layer 1
The dorsal thalamus, usually referred to simply as the thalamus, is the second-largest source of long-range excitatory inputs to neocortical L1. Contrary to the widely held view that L4, often referred to as the input layer of the cortex, is the main target of TC projections, it is now clear that most principal neurons in the thalamus (the TC or thalamic relay neurons) project to L1 and not to L4. It has been known for some time that some thalamic axons project to and branch in L1 (Herkenham 1979, Killackey & Ebner 1973, Lorente de Nó 1938). However, these projections were considered to be minor, diffuse, or nonspecific cortical inputs. It is now clear that L1-projecting TC neurons are a substantial population present throughout the thalamus.
2.2.1. Diversity of thalamocortical neurons.
The thalamus consists of several cytoarchitectonically defined nuclei, which are largely conserved across mammalian species (Jones 2007, Sherman & Guillery 2005). They include the first-order thalamic nuclei, such as the three sensory relay nuclei, the ventroposterior lateral and ventroposteromedial nuclei, the dorsal lateral geniculate nucleus, and the medial geniculate nucleus, which relay somatosensory, visual, and auditory information to the neocortex, respectively. These nuclei receive ascending sensory input and predominantly contain TC neurons that project in a topographically restricted fashion to primary cortical sensory areas in L4 and, to a lesser degree, L5b/6 [called core or C-type TC neurons by Jones (2001, 2007)]. Conversely, the less-studied higher-order thalamic nuclei, sometimes referred to as nonspecific or diffuse, primarily contain TC neurons (called matrix or M-type by Jones) that relay information from one or a few cortical areas via L5 to various layers, but mainly L1, of other cortices (Guillery 1995, Sherman & Guillery 2005).
It is now clear that most thalamic nuclei are higher-order, populated primarily by M-type TC neurons (see Rubio-Garrido et al. 2009, figure 9). They include the midline and the intralaminar nuclei, which, in addition to their diffuse projections to L1 of several cortical areas (particularly PFCs), often project prominently to the striatum (Jones 2007, Sherman & Guillery 2005, Van der Werf et al. 2002). Furthermore, there are the three higher-order nuclei primarily associated with sensory processing in the thalamus, the posteromedial thalamic nucleus (POm), the pulvinar [or lateral posterior nucleus (LP) in rodents], and the dorsal medial geniculate, which are associated with the somatosensory, visual, and auditory systems, respectively. It is well established that these nuclei are driven mainly by cortical afferents instead of ascending sensory inputs and that their projections target L1 and L5a of primary sensory cortices.
With improvements in tracing methodology and single-cell morphological analysis, it has become clear that L1-projecting TC neurons show substantial variation in the laminar distribution of their full axonal arborization, with L3, L5, and/or L6 also sometimes innervated. TC neurons also vary in the tangential spread of their cortical projections and their projections to subcortical structures. L1-projecting TC neurons in some nuclei innervate wide areas of the cortex, while those in other nuclei arborize within only one or a few adjacent cortical areas (Clasca et al. 2012, Rubio-Garrido et al. 2009). Furthermore, they differ in the sublaminar localization of the L1 projection (Figure 2). L1-projecting cells from several different thalamic nuclei can converge to the same cortical area in specific combinations.
Analyses of axons from single TC neurons show that even within the same nucleus there can be neurons with different areal and/or laminar axonal arbors. For example, while the rat POm globally projects to L1 and L5a in S1, single-cell tracing demonstrates that some POm neurons, concentrated in the anterior POm, innervate L5a more strongly than L1; others, concentrated in the posterior POm, preferentially target L1 and have wider arborizations. These results suggest that the two parts of POm may play different roles in processing somatosensory information (Ohno et al. 2012). Lastly, single-cell tracing experiments have uncovered TC neuron subtypes that project primarily to layers other than L1 or L4; for instance, some neurons in the mediodorsal (MD) nucleus have far more axons in L2/3 or L5 than in L1 (Halassa & Sherman 2019; Kuramoto et al. 2009, 2017). Molecular profiling is uncovering markers that can be used to label TC neuron subtypes (e.g., Gao et al. 2020). This approach is likely to expand in the coming years and significantly improve our understanding of the organization and function of TC communication.
2.2.2. Functional considerations.
The function of TC input to L1 is poorly understood. Hypothesized roles include arousal and brain-state control, feedback via cortico-thalamo-cortical loops, and the synchronization of spatially distributed neuronal assemblies. Until recently, it was not known whether TC projections in L1 produced weak and slow modulatory effects, as expected from their presumed areal nonspecificity, or fast and robust cortical responses like core first-order TC projections, capable of driving their postsynaptic targets. Moreover, it was unknown whether these TC projections targeted PC dendrites and/or L1 INs. Cruikshank et al. (2012; see also Anastasiades et al. 2020) found that projections from the ventromedial nucleus (VM) (and other higher-order thalamic nuclei) to the medial PFC were approximately three times more effective at exciting some L1 INs than L2/3 PCs. Interestingly, there were also differences in the short-term synaptic dynamics of the connections: Synapses with PCs showed initial facilitation during repetitive stimulation, whereas synapses with L1 INs had modest depression. These differences suggest that TC synapses in L1 are more capable of producing sustained responses on PCs during repetitive firing and that their influence might be longer lasting.
2.2.3. Other subcortical inputs to layer 1.
While the main inputs to neocortical L1 are from higher-order thalamic nuclei, neocortical areas, and neuromodulatory centers, other subcortical structures have been shown to innervate L1. Of particular interest are the zona incerta, which forms a long-range GABAergic input to L1 early in development, and the amygdala, which forms a bidirectional circuit with the PFC and recruits superficial INs (see the section titled Zona Incerta and Amygdala Inputs to Layer 1 in the Supplemental Appendix).
2.3. Inputs to Layer 1 from the Four Main Neuromodulatory Centers
Neocortical processing is conducted by local and long-range excitatory input sculpted by the activity of local GABAergic inhibitory interneurons, but it also critically depends upon the activity of four main neuromodulators: acetylcholine, noradrenaline, dopamine, and serotonin. While the laminar distribution of neuromodulatory fibers differs by cortical region and species, some projection patterns are conserved, suggesting their importance in cortical processing. Here, we highlight the contribution of neuromodulation to L1 processing across species and areas.
2.3.1. Acetylcholine.
Anatomical analysis of cortical cholinergic innervation has demonstrated an important conclusion: Despite differences in innervation patterns across cortical regions, the densest labeling is found in L1 and to a lesser degree in L4, L5, and/or L6, depending on the cortical area in rodents, cats, and monkeys (Krnjevic & Silver 1965, Lysakowski et al. 1989). These findings suggest that L1 is a critical and conserved hub for cholinergic modulation. Indeed, reports have identified L1 as the most densely innervated layer in V1 (for cats, see Aveñdano et al. 1996, Bear et al. 1985; for rats, see Mechawar et al. 2000), A1 (for monkeys, see Campbell et al. 1987), and S1 (for rats, see Mechawar et al. 2000; for mice with L4/5 as well, see Kalmbach et al. 2012). L1 is also prominently labeled in frontal and motor cortices (Aveñdano et al. 1996, Lewis 1991, Mechawar et al. 2000). In a remarkable discovery, a recent study found cholinergic neurons in the basal forebrain that specifically project to L1 (Allaway et al. 2020). The intense marbling of cholinergic fibers in L1 highlights the importance of this layer in brain state control.
2.3.2. Noradrenaline.
Studies in rats and both New and Old World monkeys have revealed species-specific differences in noradrenergic innervation. In rats, noradrenergic fibers can be found in all layers of the neocortex but are generally densest in L1 (Berger et al. 1974, Fuxe et al. 1968, Levitt & Moore 1978). Conversely, while noradrenergic fibers also exist in all layers of monkey cortex, L1 is often minimally innervated (Campbell et al. 1987, Morrison et al. 1982b). Furthermore, differences in regional innervation also exist between rats and monkeys. In rats, noradrenergic innervation is densest in the cingulate (Cg) and less so in the sensory cortices (Fuxe et al. 1968, Levitt & Moore 1978); in monkeys, innervation is dense in the Cg and, unlike in rats, also high in somatosensory cortices (Björklund et al. 1978, Morrison et al. 1982b).
2.3.3. Dopamine.
The neocortex receives dopaminergic innervation from the ventral tegmental area and substantia nigra (Thierry et al. 1973). For monkeys and rats, dopaminergic innervation is densest in frontal areas. In particular, dopaminergic innervation of the PFC is relatively high, but it is far less so in sensorimotor areas where noradrenergic input is denser (Björklund et al. 1978). Generally, in monkeys, the most densely innervated regions are targeted throughout the layers, while less innervated regions (i.e., sensorimotor cortices) display labeling predominately in L1 and/or L5–6 (Berger et al. 1988, Lewis et al. 1987); it has even been reported that L1 is the most densely innervated layer by dopaminergic fibers across all labeled cortices, even in areas that receive relatively weak innervation (Berger et al. 1988). In rats, the precise laminar innervation varies by region, but in frontal areas, L1–3 are particularly innervated (Descarries et al. 1987, Emson & Koob 1978). Interestingly, in monkey PFC at least, noradrenergic inputs target upper L1, while dopaminergic inputs target lower L1 (Lewis et al. 1987), further supporting a functionally distinct sublaminar structure of L1. In summary, although unlikely to be the predominant neuromodulatory influence in the neocortex, dopaminergic innervation, particularly in frontal cortices, targets L1 and is a conserved feature across rodents and monkeys.
2.3.4. Serotonin.
In New and Old World monkeys, serotonergic fibers from the dorsal raphe are generally found throughout the cortical layers, although their laminar distribution varies by region. In sensory cortices, serotonergic fibers are densest in L4 and deep L6, while L1 often receives the least input (Berger et al. 1988, de Lima et al. 1988, Kosofsky et al. 1984, Morrison et al. 1982a, Wilson & Molliver 1991). Compared with monkeys, rodents and cats have greater serotonergic labeling in L1 (de Lima et al. 1988). In mice, the highest serotonergic fiber densities are found in L1 of the Cg, agranular insular, frontal, and primary somatosensory cortices (Audet et al. 1988). Similarly, one recent study in mice found that serotonergic fiber labeling decreased from rostral to caudal cortices; at rostral regions, L1 was the most heavily innervated layer. Serotonergic fibers were sparser in motor, agranular insular, somatosensory, auditory, and visual cortices but still densest in L1 (Awasthi et al. 2020). Like noradrenergic input, serotonergic innervation of the neocortex differs significantly between rodents and monkeys. In rodents, the serotonergic system is likely to have more influence on L1 circuits, where fibers may powerfully control L1 interneurons by forming baskets. Lastly, all four main modulatory fibers in L1 typically run parallel to the pia (Berger et al. 1988, Lewis et al. 1987, Mechawar et al. 2000, Morrison et al. 1982b).
In summary, while long-range projections releasing neuromodulators are present throughout the column, L1 is a key target of these neuromodulators, particularly for acetylcholine (ACh).
2.4. The Interneurons of Neocortical Layer 1
While most feedback projections are glutamatergic, feedback often elicits inhibition in its target area. Therefore, knowing the inhibitory circuitry in L1 is essential to understanding top-down processing. L1 is unique among the cortical layers. It is entirely devoid of excitatory neuron somata, yet it contains GABAergic inhibitory INs at a density similar to other layers (Xu et al. 2010) (Figure 3c). Moreover, L1 lacks somata of the most-studied inhibitory neuron types in L2–6: the medial ganglionic eminence (MGE)-derived parvalbumin (PV)- and SST-expressing INs. The resident INs of L1, those with somata in L1, are instead of the less-characterized caudal ganglionic eminence (CGE)-derived, or 5HT3aR, subtypes (Tremblay et al. 2016), including several IN subtypes specialized to L1 (Schuman et al. 2019). In addition to its resident neuronal population, L1 contains a substantial proportion of the dendrites from several IN types in L2/3, including VIP and ChCs. Because these dendrites have access to the axonal projections in L1, they should be considered part of the L1 circuitry. In addition, L1 contains the ascending axon of the GABAergic SST-expressing Martinotti cells in L2/3 and infragranular layers (Figure 1).
Figure 3.
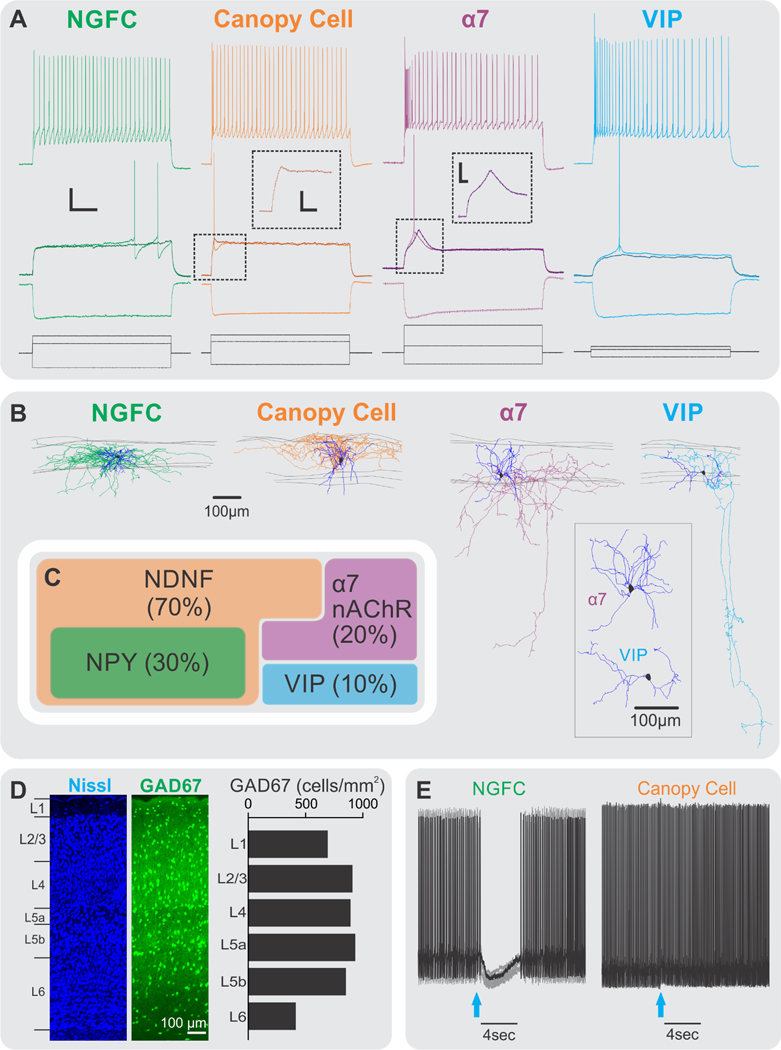
Properties of the four resident GABAergic INs of L1. The four IN subtypes (NGFCs, canopy cells, α7s, and VIPs) have unique morphological and electrophysiological properties. (a) Voltage responses of L1 INs (middle traces in color) in response to hyperpolarizing, just-subthreshold, just-threshold, and suprathreshold current injections (black; bottom traces). Insets show the near-threshold depolarizing hump in α7 cells that is absent in canopy cells. Note the near-threshold delayed spiking in NGFCs, high input resistance in VIP cells, and higher spike frequency adaption for α7 and VIP cells. Main scale bars are 20mV or 400pA and 200ms; inset scale bars are 15mV and 40ms. (b) Morphological reconstructions of the four resident IN populations. NGFCs and canopy cells have their axon largely confined to L1 spanning several columns, while α7 and VIP INs have descending translaminar axonal collaterals. Inset shows multipolar dendrites of α7 cells and bipolar dendrites of VIP cells. (c) Molecular markers segregate the four resident L1 INs. NGFCs and canopy cells both express NDNF, but NGFCs also express NPY. Non-NDNF IN populations include the α7 and the VIP INs. (d) The density of GABAergic INs in L1 is similar to that of other cortical layers. (Left) The cortical column in primary somatosensory cortex is stained for Nissl in a GAD67-GFP mouse. (Middle) GFP in the same cortical column is shown. (Right) The density of GAD67-expressing neurons across cortical layers (N = 2,656 counted cells) is shown. (e) NGFCs and canopy cells have similar morphologies (panel b) but differ in electrophysiological properties (panel a), connectivity (see text and Schuman et al. 2019), and cholinergic responses. The effects of a 30-ms puff of 20 μM muscarine (blue arrow) as the cells were depolarized to produce an approximately 5–10-Hz spike train are shown. A representative trace is shown in black, and all traces from that session are shown in gray. Abbreviations: α7, α7 nicotinic receptor–expressing interneuron; GFP, green fluorescent protein; IN, interneuron; L, layer; nAChR, nicotinic acetylcholine receptor; NDNF, neuron-derived neurotrophic factor; NGFC, neurogliaform cell; NPY, neuropeptide Y; VIP, vasoactive intestinal peptide–expressing interneuron. Panels a, b, and c modified from Schuman et al. (2019).
2.4.1. Resident layer 1 interneuron population.
Excluding the developmentally important Cajal-Retzius cells (reviewed in Marin-Padilla 1998), which disappear in rodents by postnatal day 7, most studies have agreed that there are two major morphological IN types in L1: neurons with axons largely confined to L1 and neurons with prominent axon collaterals descending to deeper layers (Hestrin & Armstrong 1996, Jiang et al. 2013, Kubota et al. 2011, Wozny & Williams 2011, Zhou & Hablitz 1996). Using electrophysiology, many studies have identified a late-spiking (LS) IN type in which positive current injections near threshold produce delayed spikes following a slowly depolarizing ramp. This LS firing pattern is characteristic of neurogliaform cells (NGFCs) in other neocortical layers and in the hippocampus (reviewed in Overstreet-Wadiche & McBain 2015, Tremblay et al. 2016). However, most L1 INs are non-LS. Although several studies have noted a diversity of firing properties among the non-LS neurons, no study has successfully divided them into discrete neuronal populations. Furthermore, while studies exploring both morphology and physiology observed that LS cells had their axon largely confined to L1, it was clear that not all cells with this morphological property were LS (Wozny & Williams 2011).
In Schuman et al. (2019), we identified molecular markers that segregated populations of cells in L1 as candidates for parsing more subtle electrophysiological and morphological distinctions. In adult mice, ~70% of L1 INs expressed neuron-derived neurotrophic factor (NDNF), and of these cells, ~40% (or 30% of total L1) also expressed neuropeptide Y (NPY), as reported by an NPY-GFP reporter mouse line. Moreover, virtually all L1 NPY-positive cells in these mice were also NDNF positive. The NDNF-negative cells could be further divided into two groups: Two-thirds expressed nicotinic α7 receptors (20% of total L1), and the other third expressed VIP (10% of total L1) (Figure 3c). Each of these molecular groups had characteristic morphological and electrophysiological properties, leading to the proposal of four resident IN subtypes (Schuman et al. 2019): NGFCs, which express NDNF and NPY; canopy cells, which express NDNF but not NPY; and α7 and VIP cells, which lack NDNF but express the α7 nicotinic acetylcholine receptor or VIP, respectively.
Morphologically, NGFCs and canopy cells both possess dense axonal arbors largely confined to L1, which extend horizontally and span several cortical columns. The horizontal extension of canopy cell axons is longer and less dense than those of L1 NGFCs. Furthermore, the axon of canopy cells is concentrated in the upper half of L1 (or L1a), while the axon of NGFCs is localized throughout L1 (Schuman et al. 2019). Electrophysiologically, NGFCs and canopy cells are quite distinct; most notably, NGFCs are LS, whereas canopy cells exhibit early-onset spikes (Figure 3a). The two NDNF cell types also differ significantly in their output connectivity (see below) and their modulation by ACh (Figure 3e). In addition, canopy cell somata are biased to L1a, while NGFCs are distributed more evenly in L1 (Schuman et al. 2019). A recent study has shown that the two NDNF cell types also receive different inputs (Ibrahim et al. 2021).
The somata of the remaining two groups (α7 and VIP cells) are biased toward the lower half of L1 (L1b). The two types are grossly similar morphologically in that both project axon collaterals to deeper layers. While both α7 and VIP cell axons reach L5a and branch there, VIP axons often continue to L6, sometimes entering the white matter (Figure 3b). In addition, the dendritic arbor of α7 cells is multipolar, while the dendrites of VIP cells are bipolar and more horizontally oriented than deeper-layer VIP bipolar dendrites (Figure 3b). Lastly, α7 somas, the largest in L1, are twice as large as VIP cell somas, the smallest in L1 (Schuman et al. 2019).
Electrophysiologically, both the α7 and VIP subtypes, like canopy cells, are non-LS with early-onset spikes. However, the three subtypes differ significantly in several properties (Schuman et al. 2019) (Figure 3a). Notably, α7 cells have a prominent depolarizing hump near threshold mediated by T-type Ca2+ channels, as in thalamic relay neurons (Schuman et al. 2019). Having established these four IN populations with a combined molecular, electrophysiological, and morphological approach, we have observed that the electrophysiological differences are usually sufficiently distinct to assign subtypes using electrophysiology alone. Canopy and α7 cells appear to be virtually exclusive to L1, and while VIP cells and NGFCs are present in other layers, in L1 they have unique properties, emphasizing the special character of this layer.1
Another distinct cell type, possibly unique to humans, has been described and named the rosehip cell, due to its large axonal boutons. They constitute 10% of the neurons in human L1, are irregular spiking, and preferentially inhibit L3 PCs (Boldog et al. 2018).
2.4.2. Connectivity of layer 1 interneurons.
An understanding of the connectivity of L1 INs will be essential for elucidating L1 circuits, but much is still unknown regarding the synaptic targets of the resident L1 IN populations. Best understood is the output connectivity of NGFCs, which have reliably demonstrated a high connection probability to most nearby cell types in the hippocampus and cortical L2/3 (Price et al. 2008, Schuman et al. 2019, Tamas et al. 2003, Wozny & Williams 2011). Furthermore, NGFCs have unique synaptic properties among GABAergic INs, including their ability to produce GABAB receptor–mediated responses in postsynaptic targets following a single presynaptic action potential (reviewed in Tremblay et al. 2016). In contrast, PV and SST INs require high-frequency firing to elicit GABAB responses (Oswald et al. 2009, Urban-Ciecko et al. 2015). NGFCs are thought to release GABA into the extracellular space via volume transmission (Oláh et al. 2009). This would explain their high probability of connection and ability to produce GABAB responses, which involve the activation of extrasynaptic GABAB receptors. NGFCs in L1 seem to share these properties, and in Schuman et al. (2019), we found a high probability of connection from L1 NGFCs to nearby L2 PCs, including GABAA receptor– and GABAB receptor–mediated responses, consistent with previous reports (Jiang et al. 2013, Oláh et al. 2009, Schuman et al. 2019, Wozny & Williams 2011). Conversely, excitatory connections from L2/3 PCs to L1 NGFCs are rare or absent (Chu et al. 2003). NGFCs can also inhibit L5 PCs, although the connectivity is less common and weaker than their connectivity to L2/3 PCs (Jiang et al. 2015, Lee et al. 2015).
Chemical synapses between NGFCs and the other resident L1 IN populations are both common and strong (with other NGFCs, see Chu et al. 2003; with canopy cells, see Schuman et al. 2019; and with non-NGFCs in general, see Chu et al. 2003 and Jiang et al. 2013, 2015). In addition, L1 NGFCs form electrical connections with each other and other INs at high rates (Chu et al. 2003, Oláh et al. 2009). Importantly, the main synaptic targets of canopy cells remain unknown. In Schuman et al. (2019), we found connections between canopy cells and L2 PCs to be significantly rarer and weaker than those made by L1 NGFCs and to lack a GABAB receptor–mediated component. Furthermore, we found a high probability of connection between canopy cells and NGFCs, in which NGFCs elicited a stronger inhibition of canopy cells than the reverse connection.
While much less is known about the connectivity of α7 and L1 VIP cells, we found that α7 cells synapse onto nearby L2 PCs with a connection probability and unitary strength between those of NGFCs and canopy cells (B. Schuman & B. Rudy, unpublished results). Jiang et al. (2015) studied the output connectivity of their single bouquet cell (SBC) population, which likely includes the α7 and VIP subtypes. They found that SBCs, compared to NGFCs, formed weaker and rarer connections with L1 INs and targeted some L2/3 IN populations (Jiang et al. 2013, 2015). Due to the horizontal extent of their axon, NGFCs inhibit INs and PCs in neighboring cortical columns, something not observed for SBCs (Jiang et al. 2013).
2.4.3. Layer 2/3 chandelier cells.
ChCs, or axo-axonic cells, are a unique subtype of GABAergic IN (reviewed in Tremblay et al. 2016) and an often-ignored component of the L1 circuit. Although they have variable PV expression, ChCs exhibit fast-spiking properties similar to PV basket cells. ChCs possess a characteristic axonal arbor with branches that terminate in vertically aligned cartridges, which target the PC axon initial segment, the site of action potential initiation. ChC somas are enriched in L2/3, but most of their dendrite is in L1. Dendritic reconstructions reveal extensive branching in L1 extending to the pia (Wang et al. 2019), and interestingly, most ChC dendritic protrusions are located within the top half of L1. This suggests that ChCs receive inputs primarily from projections that target superficial L1 (e.g., POm, VM) (Figure 2). Because ChCs can regulate action potential initiation across large ensembles of L2/3 PCs, they may represent an important conduit for L1 input to regulate L2/3 PC output.
2.4.4. Layer 2/3 VIP cells.
VIP-expressing INs are the major IN population in L2/3, representing ~55% of the total IN population (Tremblay et al. 2016). Morphologically, most VIP INs have bipolar dendrites. The ascending dendrite extends vertically to the pia and ramifies to produce a dendritic tuft. A less-frequent VIP IN type in L2/3, enriched near the L1/2 border, coexpresses CCK and has multipolar dendrites that branch in L2 and lower L1 (He et al. 2016, Pronneke et al. 2015, Tremblay et al. 2016). VIP INs as a whole preferentially target SST INs (Lee et al. 2013, Pfeffer et al. 2013), disinhibiting PC dendrites. We believe that this connectivity applies mainly to bipolar VIP INs, but the connectivity of the two L2/3 VIP IN types remains to be systematically studied (see the section titled Interneuron Circuits in Neocortical Layer 1 in the Supplemental Appendix).
2.4.5. Martinotti cells.
SST-expressing INs are powerfully recruited by local excitation through facilitating synapses; they are the main source of fast dendritic inhibition in the cortex and the main IN type mediating disynaptic feedback inhibition of PCs (Kapfer et al. 2007, Silberberg & Markram 2007; reviewed in Tremblay et al. 2016). SST INs are present in L2–6 and enriched in L5. Two major groups of SST INs have been described: Martinotti and non-Martinotti cells. Martinotti cells, present in L2/3 and infragranular layers, have an axon that extends vertically through the cortical layers, branching extensively and spreading horizontally in L1. Non-Martinotti cells have been described in somatosensory cortex and are present in L4 and L5; they lack an ascending axon to L1 and instead innervate L4 (Muñoz et al. 2017, Tremblay et al. 2016, Xu et al. 2013). There is evidence for the existence of diverse Martinotti cells that differ in the laminar and sublaminar (within L1) distribution of their ascending axon (Muñoz et al. 2017), which may contribute to the specificity of inhibition of PC dendrites by distinct inputs (see Section 2.5). Unlike other IN subtypes, SST INs do not inhibit each other. As a result, their activity increases proportionally to the activity in the excitatory network, and due to the morphology of the Martinotti cell axon, their influence can spread to several columns. It has been proposed that SST INs mediate surround suppression, a function that results from these unique properties (Adesnik et al. 2012; reviewed in Tremblay et al. 2016).
Martinotti cells are likely the main mediators of inhibition to the PC tuft dendrites in response to local excitation, while L1 and L2/3 INs (other than SST INs) are the main mediators of inhibition elicited by long-range excitation. Consistent with this, Murayama et al. (2009) found that burst firing in PCs disynaptically inhibited dendritic Ca2+ signals in the same or neighboring PCs. Conversely, dendritic inhibition by contralateral axons mediating interhemispheric inhibition depends on L1 INs (Palmer et al. 2012). Furthermore, while local recruitment of Martinotti cells likely produces GABAA receptor–mediated inhibition of PC dendrites, interhemispheric inhibition depends on GABAB receptors (Murayama et al. 2009, Palmer et al. 2012).
2.4.6. Layer 1 interneuron circuits.
To better understand the circuits that mediate L1 processing, we will need to better understand the output connectivity of L1 INs and the synaptic targets of different L1 inputs. Nonetheless, based on our current knowledge, it is clear that L1 circuitry can mediate both inhibition and disinhibition of PC dendrites. Three L1 IN circuits have been studied: a disinhibitory circuit mediated by VIP INs, a GABAB NGFC-mediated feedforward inhibitory circuit, and a Martinotti cell–mediated inhibition of NDNF INs (see the section titled Interneuron Circuits in Neocortical Layer 1 in the Supplemental Appendix).
2.5. Dendritic Tufts of Pyramidal Cells in Neocortical Layer 1
Most neocortical neurons are excitatory [>80% in rodents (Meyer et al. 2011)], and the output of the neocortex is predominantly excitatory. The main output cells of the neocortex are the PCs, whose axons project to many distant and local regions. L1 inputs must have an impact on PC activity to influence the cortex. One of the hallmarks of L1 is that it is devoid of excitatory cell somata. However, PCs extend their apical dendrites into L1, where they ramify into tufts of small-diameter dendrites heavily studded with spines. This significant increase of surface area suggests that PCs are capable of sampling substantial input to L1, and there is evidence that PCs are indeed targets of L1 inputs (e.g., Cauller & Connors 1994, Gambino et al. 2014, Ibrahim et al. 2016, Lee et al. 2013, Manita et al. 2015, Petreanu et al. 2009, Xu et al. 2012).
Due to the cable properties of thin processes, synaptic potentials generated in distal dendrites are expected to be attenuated and filtered as they propagate to the soma and the axon initial segment. Indeed, dual somatic and dendritic recordings have shown that voltage attenuation along the dendrites is considerable (Stuart & Spruston 1998, Williams & Stuart 2002; reviewed in Stuart & Spruston 2015). This raises the following question: How can distal L1 synaptic inputs influence the action potential output of PCs? Thus, the discovery that dendrites have conductances that produce active electrogenesis or dendritic spikes, found first in cerebellar Purkinje cells by Rodolfo Llinás and then in PCs of the neocortex and hippocampus, is of major significance and explains how L1 inputs at the distal dendrites can influence the timing and probability of PC output (reviewed in Häusser et al. 2000, Magee 2000, Major et al. 2013, Stuart & Spruston 2015).
Three main types of dendritic spikes have been described in cortical PCs (reviewed in Häusser et al. 2000, Larkum 2013, Magee 2000, Major et al. 2013, Stuart & Spruston 2015). (a) Ca2+ spikes are generated in the apical dendritic trunk, near the primary bifurcation of the apical dendrite, and are mediated by Ca2+ influx through voltage-gated Ca2+ channels. Depolarizing input to the distal apical dendrite activates these Ca2+ channels and, if of sufficient strength and duration, produces a prolonged (>10 ms) depolarization or plateau potential (Larkum et al. 2001). (b) Dendritic Na+ spikes are generated by local voltage-gated Na+ channels in the thin apical and basal dendrites and can occur in the absence of axonal and somatic action potentials [as is the case for the Ca2+ spikes described above and N-methyl-d-aspartic acid (NMDA) spikes described below]. They are broad compared to somato-axonal action potentials but narrower than Ca2+ spikes (Harnett et al. 2013, Larkum et al. 2009). (c) NMDA spikes are mediated by local NMDA receptor channels in the PC tuft dendrites and can be generated in basal dendrites as well (Larkum et al. 2009). NMDA spikes are even longer in duration than Ca2+ spikes and are more compartmentalized. Since NMDA receptors require bound glutamate, NMDA spikes are thought not to propagate actively from the activated input site like Na+ and Ca2+ spikes do, and they can occur in specific tuft branches and poorly invade neighboring branches. Inactivating or A-type K+ channels (presumably Kv4-mediated K+ channels, which are enriched in the tuft dendrites of neocortical PCs) also limit the spread of NMDA spikes, which can be enhanced by depolarization of the dendritic branch (Harnett et al. 2013). NMDA spikes in the tuft dendrites have been shown to have a large influence on the output of L2/3 PCs in response to sensory stimuli (Palmer et al. 2014).
In addition to these dendritic spikes, dendritic recordings have provided evidence that somato-axonal action potentials actively back-propagate into the dendritic tree of cortical PCs. Back-propagating action potentials (bAPs) can interact with synaptic inputs and locally generated depolarizations, further amplifying and facilitating dendritic spiking in PCs (Larkum 2013, Stuart & Spruston 2015, Stuart et al. 1997).
These mechanisms can work independently or in different combinations to potentiate weaker input to very distal dendrites, profoundly and differentially altering PC output. For instance, in the model of PCs as coincidence detectors (Larkum 1999, 2001, 2013), relatively weak and short input to L5 PC distal apical dendrites produces an excitatory postsynaptic potential, which propagates with decrement to the soma, but does not elicit a dendritic Ca2+ spike (stronger or longer-lasting inputs to the same dendrite might alone generate these spikes). In the same cell, input close to the soma generates a somato-axonal spike that back-propagates to the dendrite, but the bAP is also insufficient to generate a Ca2+ spike in the distal dendrite. However, if apical dendritic input coincides with input close to the soma, the dendritic depolarization interacts with the bAP, and the depolarization of the dendrite is increased in amplitude and duration. This produces a dendritic Ca2+ spike, which efficiently drives the PC soma, generating high-frequency bursts of action potentials.
Other examples of coincidence detection have been described as well. Williams & Stuart (2002) showed that nearly synchronous inputs (within ~10 ms) at the same dendritic location could generate dendritic spikes and directly influence action potential output. A study by Polsky et al. (2004) emphasized the importance of synapse location: Nearby inputs on the same branch summed sigmoidally, whereas widely separated inputs or inputs to different branches summed linearly. Furthermore, not only the generation but also the propagation of dendritic spikes to the soma can be facilitated by proximal inputs (Jarsky et al. 2005, Stuart & Spruston 2015, Takahashi & Magee 2009).
Inhibition can play a major role in integrating synaptic inputs at the apical dendrites, amplifying the complexity of dendritic integration. Two groups of inhibitory cells have already been shown to affect Ca2+ signals in the distal dendrites of PCs: SST-expressing Martinotti cells and NDNF-expressing L1 INs (Abs et al. 2018, Cichon & Gan 2015, Murayama et al. 2009). These and additional studies illustrate the enormous capability of complex computations at the cellular level in PCs. The challenge now is to discover how they are used during behavior to mediate top down modulation of PC activity.
2.5.1. Plasticity and dendritic signaling.
The ion channels that mediate dendritic excitability have activity-dependent dynamics and are regulated by neuromodulators, which can affect dendritic signaling. Furthermore, in addition to the electrical effects on spiking of the dendritic spikes, the local increases in intracellular Ca2+ through Ca2+ channels or NMDA receptors can mediate Ca2+-dependent signaling cascades important for plasticity (e.g., Frick & Johnston 2005, Gambino et al. 2014, Golding et al. 2002, Letzkus et al. 2006, Losonczy et al. 2008, Magee & Johnston 1997, Mago et al. 2020, Sjöström & Häusser 2006, Weber et al. 2016, Williams & Holtmaat 2019). The strengthening or weakening of synapses through these mechanisms can be important for the generation of sensory representations and mediate effects of top-down modulation on learning (see Section 3).
2.5.2. Diversity and specificity of dendritic integration.
Cortical PCs are the executing element in a variety of circuits, making it unlikely that all L1 inputs are processed the same by every PC (e.g., Kim et al. 2015). L1 contains long-range inputs capable of providing global, state-dependent information as well as area-specific feedback to PC apical dendrites. To ensure the proper integration of these diverse long-range inputs into a given cortical area during the engagement of local PC ensembles, apical dendrites should be capable of discriminating between different inputs. The differences in the laminar and sublaminar distribution of distinct projections described earlier can underlie differences in afferent connectivity to PC dendritic branches (including whether inputs are dispersed among different branches or clustered on one, which can affect the generation of dendritic spikes) (Kerlin et al. 2019, Stuart & Spruston 2015). Differences in synaptic dynamics and synaptic strengthening of distinct inputs through plasticity, neuromodulation, and the intrinsic morphological and electrophysiological properties of individual dendrites also likely contribute to the mechanisms by which distinct PC ensembles respond to different inputs. Lastly, differences in morphology or electrical properties of the dendritic arbor of distinct PCs could contribute to the specificity of actions of L1 inputs. This has not been extensively documented, but interesting examples have been described (see the section titled Diversity of Pyramidal Cell Dendrites and Dendritic Integration in the Supplemental Appendix).
Considered together, these lines of research indicate that the complex filtering properties of PC dendrites can play crucial roles in diversifying L1 input processing.
3. LAYER 1 AND NEOCORTICAL FUNCTION
Given that L1 receives a large proportion of the connections between cortical areas and from subcortical structures, it is expected to have major roles in cortical function. Feedback connections have been theorized to gate inputs and convey expectations and context as well as mediate states of consciousness, attention, cross-modal interactions, sensory perception, and learning (Bastos et al. 2012, Gilbert & Li 2013, Gilbert & Sigman 2007, Heeger 2017, Keller & Mrsic-Flogel 2018, Larkum 2013, Zagha 2020). While the literature addressing top-down processing and its possible functions is vast, our understanding of the underlying mechanisms is still poor. Here, we first discuss two distinct models of neocortical operation, which make different assumptions about the function of top-down processing. Then we highlight some of the studies that provide evidence for the involvement of L1 processing in several neocortical functions. While neuromodulators unquestionably impact these functions, we do not discuss their role because their actions on L1 circuitry are largely unknown.
3.1. Representational Versus Predictive Frameworks of Cortical Function
There are two distinct views of what the neocortex does. According to one view, sometimes referred to as the representational framework, which has dominated the thinking of brain scientists over the last 75 years, the activity of neurons in sensory areas is a representation of a feature or object in the environment. Starting with the feedforward sensory input and using a hierarchical structure, the neocortex constructs an increasingly complex neuronal representation of sensory information. Perception depends on activity in neurons (called feature detectors) tuned to this representation of the world. The role of feedback in this framework is to modify, or modulate, bottom-up signals and thus contribute to the construction of the best sensory representation for particular behavioral needs.
At the other extreme (with many variants in between) is the view that at the heart of perception are models or predictions about what exists in the environment, internally generated based on past sensory experience and the results of our actions. At a given moment, perception is a comparison between the internal prediction and the sensory input. Although this view traces its origins to ideas developed by von Helmholtz in the 1860s (Bastos et al. 2012, Friston 2018, Keller & Mrsic-Flogel 2018), the view that the brain uses Bayesian inference to generate percepts has been gaining proponents in recent decades, and several models have been proposed. We focus on one of these models, initially proposed by Rao & Ballard (1999; Bastos et al. 2012, Keller & Mrsic-Flogel 2018), known as predictive coding. Variants of this model are particularly attractive because neural substrates for how the brain implements these models have been proposed, so it is possible to envision circuit mechanisms and generate testable hypotheses. Conversely, for the representational framework, there are no specific hypotheses as to what feedback does to improve the sensory representation, other than providing attentional and learning signals and gating specific inputs, all of which are accepted to occur in both the predictive coding and representational frameworks.
According to predictive coding models, top-down or feedback inputs from a higher level of the hierarchy send to lower levels a prediction of their bottom-up input (the sensory input in primary sensory cortices). The prediction and the bottom-up input are compared, and the difference, a prediction error, is then forwarded to the higher area to update the prediction. At the next station in the hierarchy, the prediction error is the feedforward signal and is compared to the feedback prediction from a higher cortical area to update the internal model, and so forth. Predictions are thought to arise from L5 PCs and prediction errors from L2/3 PCs. Moreover, predictions are thought to elicit inhibition (Bastos et al. 2012) or both inhibition and excitation in the target neurons (Keller & Mrsic-Flogel 2018).
That the brain makes predictions and that perception involves prediction are largely accepted. It explains why our mind can finish a sentence before the speaker has uttered all the words; why we miss things that are right in front of our eyes, such as failing to see major errors in a text after rereading it multiple times; why we almost feel things we see or see things we hear; and many other everyday occurrences. In fact, most would accept that predictions and expectations are part of the information contained in feedback signals; what is controversial is the specific set of ideas in predictive coding models as well as the view that what is computed and fed forward are prediction errors. Predictive coding models are an extension of attempts to explain why we do not see a moving world when our eyes move or why we do not hear the sounds produced by our own movements. To explain these phenomena, it has been suggested that a copy (efference copy) of the respective motor-related signals produces a corollary discharge, a prediction of the self-generated sensory stimuli. This prediction is relayed to the appropriate sensory system, where it suppresses the neural responses to the sensory stimuli generated by self-movement (Schneider & Mooney 2018, Schneider et al. 2014). Some would argue that the neocortex uses both the representational and the predictive frameworks in different situations or depending on the feedback.
The predictive coding model we have been discussing was initially proposed to explain observed nonclassical receptive-field phenomena [e.g., endstopping (see Rao & Ballard 1999)]. Recent experiments to test predictive coding are based on the idea that when sensory stimuli become predictable (e.g., by repetitively presenting the same stimulus), the response in prediction error neurons should decrease. If the stimulus is then unexpectedly removed or changed, the response should increase. Both of these have been observed in mice, humans, and monkeys (Fiser et al. 2016, Keller et al. 2012, Schwiedrzik & Freiwald 2017, Stanley & Miall 2007). Although our discussion was focused on perception, as is much of the research on predictive coding, it is thought that the same model could apply throughout the brain.
Because predictive coding may require very precise connectivity of feedback projections with prediction error neurons, it poses a topography challenge. The prediction signals relayed by the feedback axons need to target populations of neurons (presumably L2/3 PCs) that receive the appropriate bottom-up signal (for instance, the appropriate receptive field selectivity) for proper comparison. Marques et al. (2018) compared the receptive field properties of feedback axons from the lateromedial visual cortex in L1 of V1 with those of the underlying L2/3 PCs and concluded that feedback inputs show overall tuning-dependent retinotopic specificity (see also Keller et al. 2020). While this is a good start, one needs to consider the connectivity of the INs in L1 that may mediate inhibitory or disinhibitory signals to the L2/3 PCs. This may require patterns of connectivity of INs that are unlike the highly divergent connectivity usually assumed for these cells (Tremblay et al. 2016). An interesting solution to the topography problem is to propose that connectivity is promiscuous; the proper functional connections are established by experience (e.g., by strengthening the synapses that reduce the prediction error) (D. Schneider, personal communication).
3.2. Neocortical Layer 1 and Sensory Perception
Several lines of intriguing evidence suggest that feedback projections play a crucial role in conscious perception. The somatosensory-evoked potential, as measured in primate S1, is a field potential with two main components: an early (~12 ms poststimulus) surface-positive signal (P1) whose amplitude correlates with stimulus intensity and a later (~50 ms poststimulus) surface-negative signal (N1) whose amplitude correlates with the reported discrimination of somatosensory stimuli (i.e., higher-intensity touch stimulation that is reported more quickly accompanies greater N1 amplitudes) (Cauller & Kulics 1988, 1991). While the P1 component is due to synaptic input in L4, the N1 component is generated by synaptic excitation in L1, suggesting that its origin is from feedback fibers. Furthermore, the N1 component is severely attenuated or absent in unconscious animals (e.g., sleep, anesthesia) (Arezzo et al. 1981, Cauller & Kulics 1988). These two pieces of evidence suggest that L1 may either receive or partly generate a signal via feedback input that is important for conscious perception.
3.2.1. Corticocortical feedback in sensory perception.
As discussed above, innervation of L1 by corticocortical feedback from higher to lower areas in a hierarchy is a ubiquitous motif in the structure of cortical circuits. However, while there is a wealth of anatomical evidence for such connections, the information they convey is largely unknown. Nonetheless, several studies have demonstrated that corticocortical feedback fibers in L1 carry, as expected, more processed sensory information than does bottom-up input from lower areas. This feedback input may facilitate the processing of sensory information earlier in the hierarchy and therefore expedite behavioral responses (see the section titled Informational Content of Corticocortical Feedback in Layer 1 in the Supplemental Appendix).
Several recent papers, mainly in rodents, have begun to provide evidence that L1 processing, particularly Ca2+ signals in the distal apical dendrite, is important for sensory perception. Using an object-localization task, Xu et al. (2012) observed global Ca2+ signals in the tuft dendrites of L5 PCs when active whisker touch occurred at particular locations of the object. These signals required both sensory input and feedback input from motor cortex, suggesting that they result from the interaction of bottom-up and top-down signals. More recently, Manita et al. (2015) explored a bidirectional circuit from S1 to M2, which projects back to S1 in superficial and deep layers. They found that sensory stimuli activated S1 and then M2 before subsequently activating S1 again (Manita et al. 2015). This second excitation in S1 was likely mediated by M2 inputs and produced Ca2+ spikes in the distal apical dendrite and somato-axonal burst spiking in L5 PCs. Moreover, optogenetic inhibition of M2 feedback fibers both decreased L5 PC firing and perturbed sensory perception. Lastly, Takahashi et al. (2016, 2020) showed that Ca2+ signals in the distal apical dendrites of L5 PCs correlated with the threshold for stimulus detection in a whisker touch behavioral task, and perturbation of this activity, particularly in pyramidal tract PCs projecting to the POm, striatum, and superior colliculus, impaired the perceptual threshold.
A recent interesting study showed that feedback in V1 leads to the generation of a second receptive field in L2/3 PCs (termed fbRF) surrounding the main feedforward receptive field. Given the location of feedback fibers in L1, the generation of this fbRF likely involves L1 processing (Keller et al. 2020).
3.2.2. Thalamocortical feedback in sensory perception.
TC feedback is ideally positioned to gate or amplify top-down corticocortical connectivity. Cortico-thalamo-cortical loops are formed when sensory, motor, or frontal areas project to higher-order thalamic nuclei, which in turn project back to the cortex, predominately in L1. However, like corticocortical feedback, the function and mechanisms of TC feedback are unclear.
One source of TC feedback that has received much attention is the rodent POm, a higher-order thalamic nucleus that receives input from somatomotor cortices, including S1 (Harris et al. 2019), to which it provides feedback in L1 and L5a. Work by Mease et al. (2016) in anesthetized mice showed that optogenetic stimulation of POm fibers at the cortical surface caused stronger and more prolonged touch responses in the L5 PCs of S1, and Zhang & Bruno (2019) observed similar effects on L2/3 PCs in anesthetized or awake, mildly sedated mice, suggesting that POm–S1 feedback enhances sensory responses. Similarly, in anesthetized primates, activation or inhibition of the pulvinar (the visual equivalent of the POm) enhanced or suppressed visual responses in V1 L2/3 neurons, respectively (Purushothaman et al. 2012).
Roth et al. (2016) explored the information conveyed to L1 of V1 by the LP, a higher-order thalamic nucleus and the rodent pulvinar homolog. Using Ca2+ imaging of LP axons in L1 of V1 while presenting visual stimuli, they found that LP axons had less direction selectivity and orientation selectivity and larger, more scattered receptive fields than did underlying V1 neurons. Moreover, some LP boutons responded to self-generated movement and others to visual flow (in virtual corridors). Interestingly, some LP boutons actually conveyed the magnitude of difference between visual flow and running speeds, suggesting that LP conveys both error and agreement signals to V1.
TC feedback has also been implicated in the delay activity responsible for working memory (Bolkan et al. 2017, Guo et al. 2017) and as a means to transmit influence to neocortex from other subcortical structures such as the superior colliculus or the basal ganglia (Gharaei et al. 2020, Morrissette et al. 2019).
In summary, both corticocortical and TC feedback convey more refined information to L1 of lower cortical areas, including the earliest points of cortical processing (i.e., S1, V1, etc.). Exactly how this information is integrated into local circuits via L1 is not known, nor is it clear in many cases whether feedback produces inhibition, excitation, or a highly tuned, spatiotemporally precise combination of both. However, we now know enough to say that hypotheses first made from anatomical analysis, namely that feedback via L1 is vitally important for sensory perception, are almost certainly correct.
3.2.3. Layer 1 and cross-modal interactions.
The appropriate association of different sensory modalities is essential for perception and behavior. Increasing evidence demonstrates that, in addition to the integration of sensory modalities in higher-association areas, cross-modal interactions occur in early sensory areas (Cappe & Barone 2005, Clavagnier et al. 2004, Kayser et al. 2005, Stehberg et al. 2014). Ibrahim et al. (2016) showed that A1 projects to L1 of V1, contacting non-VIP L1 INs and likely mediating sound-induced sharpening of orientation selectivity in L2/3 PCs. Because this effect was more pronounced at lower-contrast visual stimuli, the authors suggested that cross-modal input may enhance detection of otherwise imperceptible stimuli. Work by Chou et al. (2020) demonstrated that a superior colliculus to LP to A1 circuit, mediated by L1 INs, sharpens tuning curves of L2/3 PCs to tones while increasing the signal-to-noise ratio. Furthermore, looming visual stimuli (e.g., an expanding dark disk in the upper visual field) enhanced the signal-to-noise ratio of auditory-evoked responses and sharpened auditory tuning curves, suggesting that predator detection in the cortex may utilize cross-modal input to L1.
3.3. Control of States of Consciousness, Arousal, and Awareness
A number of observations in humans and other animals suggest that top-down mechanisms and L1 have important roles in the control of states of consciousness (Llinas & Ribary 2001, Mashour 2014). In particular, studies have implicated higher-order thalamic nuclei, many of which prominently project to L1 (see Section 2.2). Several L1-targeting higher-order thalamic nuclei diffusely project to many cortical areas, suggesting their importance in global phenomena such as the control of brain states (Chen et al. 2015, Kinomura et al. 1996, Llinas & Ribary 2001, Steriade 1996, Steriade et al. 1990, Van der Werf et al. 2002). However, until recently, direct evidence that L1 processing is involved in the control of states of consciousness, or that identified L1-projecting TC neurons play such roles, was lacking.
In a recent study, Honjoh et al. (2018) used silicon probes to record the activity of TC neurons in the VM. These neurons send axons to widespread cortical areas and mainly innervate upper L1 (see Section 2.2 and Figure 2b). The activity of VM neurons was high during awake states and rapid eye movement sleep but low during non-rapid eye movement sleep. Interestingly, the increase in activity at the sleep-to-wake transition occurred seconds before cortical activation [as detected by electroencephalogram and before mice-initiated movement (based on electromyography), suggesting a causal effect. Optogenetic stimulation of VM neurons awoke mice from non-rapid eye movement sleep and caused electroencephalogram desynchronization. Furthermore, the duration of the awake period correlated with the duration of the active state of the opsin and therefore the activation of VM neurons. On the other hand, chemogenetic inhibition of VM TC neurons using the clozapine N-oxide–designer receptor exclusively activated by designer drugs system decreased the duration of awake periods. This study provides powerful evidence of a role for VM TC neurons in cortical activation and behavioral arousal.
A recent study used channelrhodopsin to specifically depolarize the distal apical dendrite of L5 PCs to imitate the effect of L1 feedback while recording somatic responses electrophysiologically (Suzuki & Larkum 2020). Suppression of conscious states using general anesthetics was found to almost completely eliminate somatic responses. Recording at different dendritic depths showed that this effect was associated with a failure of propagation of dendro-somatic signals at the bottom third of the apical dendrite. Interestingly, blockade of muscarinic or metabotropic glutamate receptors, as well as inhibition of POm, produced a similar decoupling of the dendrite and soma.
3.4. Layer 1 Circuits and Attention
Top-down, or selective, attention is thought to be a distributed process that involves a network of interconnected cortical and subcortical structures (Bolkan et al. 2017, Guo et al. 2017, Schmitt et al. 2017). Ultimately, it is believed, attentional signals from this network affect processing in lower sensory areas, enhancing neuronal responses to behaviorally relevant stimuli and, perhaps via INs, suppressing responses to distractors (Chen et al. 2015, Desimone & Duncan 1995, Gilbert & Sigman 2007, Halassa & Kastner 2017). In fact, top-down attention is often considered a key function of feedback in both the representational and the predictive frameworks by selectively gating subsets of local neuronal inputs in lower sensory areas. Enhanced responses to attended stimuli have been reported in visual cortex neurons of humans and monkeys (Desimone & Duncan 1995, Kastner & Ungerleider 2000). Evidence is now emerging that L1 circuits are involved in several stages of this process.
Frontal and parietal cortices have been implicated among the sources of top-down signals that underlie attention-specific filtering in sensory areas. Zhang et al. (2014) investigated mechanisms of attention in the mouse visual system. Optogenetic stimulation of Cg axons in V1 enhanced the firing rate of V1 neurons in response to visual stimuli of preferred orientations, similar to the effects of top-down attention. Moreover, Cg activation improved performance in a visual discrimination task. Additionally, Zhang et al. (2014) observed that stimulation of Cg axons at 200 μm from a recorded cell decreased the tuning curve amplitude, producing a facilitatory center and a suppressive surround, similar to top-down attention in primates. In slice experiments, optogenetic stimulation of Cg axons in L1 produced excitatory postsynaptic currents in L2/3 PCs and disynaptic inhibitory postsynaptic currents, which were stronger with light stimulation 200 μm away from the recorded cell rather than just above. Additional experiments revealed that Cg excitation of SST INs and VIP IN–mediated disinhibition were likely responsible for the suppressive surround and center facilitation, respectively. This circuit profile is consistent with the morphology of VIP bipolar INs, which have horizontally narrow and vertically oriented axonal arbors, and the morphology and connectivity of SST INs, which have horizontally extended axons in L1 and lack connectivity to each other (Tremblay et al. 2016).
Schmitt et al. (2017) found that the MD higher-order thalamic nucleus, which heavily projects to PFC, sustains representations of rules guiding goal-directed attention. In particular, MD projections increased the connectivity of PFC locally, which generated rule-specific neuronal sequences. It is not clear whether the observed effects are due to L1- or L3-projecting MD neurons.
TC, but also corticocortical, interactions have also been suggested to have a role in the synchronization of spatially remote assemblies of cortical neurons, a function that might be important in cognition and memory (Jones 2001, 2009; Rubio-Garrido et al. 2009; Saalmann et al. 2012; Vicente et al. 2008).
3.5. Layer 1 Circuits and Learning
Learning is a key function of the neocortex and one in which top-down processing likely plays an important role. Regardless of whether the brain utilizes predictive coding or representational models, all theories of cortical function require that internal representations be acquired and updated by experience. Given the abundant evidence of synaptic plasticity in PC tuft dendrites and that L1 is a major site for cortical interactions, L1 is well poised to have important roles in learning. Evidence for important roles of top-down modulation in learning have been reviewed elsewhere (Bastos et al. 2012, Gilbert & Sigman 2007, Keller & Mrsic-Flogel 2018); here, we discuss some of the evidence for the involvement of L1 circuitry in different types of learning.
A number of studies have imaged the activity of either corticocortical or TC feedback axons during learning tasks. Tanaka et al. (2018) imaged TC axons in L1–3 of motor cortex in mice engaged in a motor task: pushing and holding a lever for a specified duration. They found that the axons in L1 tracked specific parts of the behavior and that their activity evolved as the task was learned. In another study, Leinweber et al. (2017) imaged the activity of feedback projections from Cg and motor cortex to V1 of mice learning to navigate a two-dimensional virtual environment. Axonal activity was found to correlate with locomotion and visual flow feedback; by recording during different training days, they observed that the signals changed depending on visuomotor experience. Learning-associated changes in the Ca2+ signals of the PC tuft dendrites in S1 or motor cortex have also been reported (Cichon & Gan 2015, Lacefield et al. 2019).
Doron et al. (2020) reported evidence for the involvement of L1 in a hippocampal-dependent associative learning task. Mice were trained to report microstimulation of S1, a task previously shown to be hippocampal dependent. Chemogenetic suppression of the dense L1 projection from perirhinal cortex to vibrissal S1 disrupted memory formation. Interestingly, there was no effect of the manipulation in mice that had already learned the task. Furthermore, Doron et al. reported that the learning was associated with the emergence of a small (~10%) population of L5 PCs that fired high-frequency spike bursts and had enhanced dendritic excitability. Dendritic events and bursting were also inhibited by the chemogenetic suppression of the perirhinal input.
Some studies have directly implicated L1 INs in associative learning. For instance, Letzkus et al. (2011) studied the mechanisms of auditory associative fear conditioning in mice. They found that foot shocks or tones paired with foot shocks activated L1 INs via a nicotinic mechanism in the auditory cortex. These INs inhibited L2/3 PV INs, resulting in the disinhibition of L2/3 PCs. They reported that pharmacological or optogenetic block of the disinhibition diminishes fear learning. More recently, the same group (Abs et al. 2018) reported increased responses to the conditioned auditory stimuli in NDNF INs. How disinhibition of the soma and inhibition of the dendrites contribute to the storage of the association remains to be investigated.
4. PERSPECTIVES
The account presented here illustrates the amazing richness of the information that arrives in neocortical L1 to mediate top-down processing, as well as the astonishing complexity of the neuronal elements in L1. With at least six or seven IN subtypes positioned to process long-range inputs, L1 contains a diversity of GABAergic INs that far surpasses that in other layers. Moreover, L1 contains the axonal arbors of diverse SST-expressing Martinotti cells that can differentially influence the activity of local INs and PC tuft dendritic branches. This landscape contrasts with L4, the main recipient layer of bottom-up input, which contains only three or four IN subtypes (Beierlein et al. 2003, Chittajallu et al. 2013, Tremblay et al. 2016). In addition, L1 contains the fine apical tuft dendrites of PCs that are endowed with a complex anatomy and a rich repertoire of conductances, capable of producing highly elaborate electrical and chemical transformations of the signals they receive. As we illustrate in this review, L1 processing is thought to be involved in functions ranging from sensory perception and learning to controlling states of consciousness and attention. We hope the examples discussed here illustrate the importance of signal processing in L1.
Our understanding of the circuit mechanisms by which the components of L1 process top-down input is still in its infancy. In this journal, David Hubel (1982) called L1 the crowning mystery of the cortex. However, since then, as documented here, great progress has been made in understanding the cellular components of L1 and the mechanisms by which inputs to the distal tuft dendrites in L1 control the magnitude and timing of PC output. Considering these advances and major improvements in electrophysiological and genetic methods that allow unprecedented study of neuronal function in behaving animals and manipulation of neuronal circuits, we expect significant progress in coming years toward understanding how top-down and bottom-up information are integrated in the neocortex. While L1 is the main target of feedback projections, many projections also include significant targeting of other layers, often either L5a or L6 (see Section 2). The repetition of these patterns is intriguing, but how L1 processing is integrated with these other layers remains to be discovered.
Supplementary Material
SUMMARY POINTS.
Neocortical function critically depends upon a hierarchical structure and the integration of internally generated top-down and sensory-driven bottom-up signals.
Neocortical layer 1 (L1) is the predominant input layer for top-down information, relayed by a rich plexus of corticocortical projections, thalamocortical projections from higher-order thalamic nuclei, and neuromodulatory projections.
Top-down and bottom-up information are integrated in the pyramidal cells (PCs) in layer 2/3 and layer 5, which utilize two electrotonically segregated compartments to optimize this integration: the apical tuft dendrites in L1 that receive top-down inputs and the basal dendrites and somatic/axonal area that receive bottom-up signals.
The tuft dendrites are capable of highly complex nonlinear processing critical for the integration of top-down and bottom-up information.
L1 function depends on a remarkably complex network of diverse GABAergic interneurons that mediate inhibition or disinhibition to gate the flow of top-down information with specific spatiotemporal patterns and sculpt how it is delivered to the distal dendritic branches of PCs.
L1 plays important roles in cortical functions, including sensory perception, cross-modal association, and controlling states of consciousness, attention, and learning.
ACKNOWLEDGMENTS
This work was supported by National Institutes of Health grants P01NS074972, R01NS107257, and R01NS110079 to B.R. and F31NS106793 to B.S. The authors thank Manuel Valero, David Schneider, Daniel Levenstein, Edward Zagha, Soohyun Lee, Judit Makara, Gord Fishell, and members of the Fishell and Rudy labs for their helpful discussion, comments, and advice. We also thank Yoshiko Hashikawa for her contributions to this work.
DISCLOSURE STATEMENT
The authors are not aware of any affiliations, memberships, funding, or financial holdings that might be perceived as affecting the objectivity of this review.
Footnotes
Using morphological criteria, Jiang et al. 2013, 2015) classified L1 neurons into elongated neurogliaform cells and single bouquet cells (SBCs), which were characterized by having vertically descending axon collaterals. SBCs likely include both the VIP and α7 cells.
LITERATURE CITED
- Abs E, Poorthuis RB, Apelblat D, Muhammad K, Pardi MB, et al. 2018. Learning-related plasticity in dendrite-targeting layer 1 interneurons. Neuron 100:684–99.e6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adesnik H, Bruns W, Taniguchi H, Huang ZJ, Scanziani M. 2012. A neural circuit for spatial summation in visual cortex. Nature 490:226–31 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allaway KC, Muñoz W, Tremblay R, Sherer M, Herron J, et al. 2020. Cellular birthdate predicts laminar and regional cholinergic projection topography in the forebrain. eLife 9:e63249 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anastasiades PG, Collins DP, Carter AG. 2020. Mediodorsal and ventromedial thalamus engage distinct L1 circuits in the prefrontal cortex. Neuron 109:314–30.e4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arezzo JC, Vaughan HG Jr., Legatt AD. 1981. Topography and intracranial sources of somatosensory evoked potentials in the monkey. II. Cortical components. Electroencephalogr. Clin. Neurophysiol 51:1–18 [DOI] [PubMed] [Google Scholar]
- Audet MA, Doucet G, Oleskevich S, Descarries L. 1988. Quantified regional and laminar distribution of the noradrenaline innervation in the anterior half of the adult rat cerebral cortex. J. Comp. Neurol 274:307–18 [DOI] [PubMed] [Google Scholar]
- Aveñdano C, Umbriaco D, Dykes RW, Descarries L. 1996. Acetylcholine innervation of sensory and motor neocortical areas in adult cat: a choline acetyltransferase immunohistochemical study. J. Chem. Neuroanat 11:113–30 [DOI] [PubMed] [Google Scholar]
- Awasthi JR, Tamada K, Overton ETN, Takumi T. 2020. Comprehensive topographical map of the serotonergic fibers in the mouse brain. J. Comp. Neurol https://doi.org/0.1002/cne.25027 [DOI] [PubMed] [Google Scholar]
- Barbas H, Pandya DN. 1989. Architecture and intrinsic connections of the prefrontal cortex in the rhesus monkey. J. Comp. Neurol 286:353–75 [DOI] [PubMed] [Google Scholar]
- Bastos AM, Usrey WM, Adams RA, Mangun GR, Fries P, Friston KJ. 2012. Canonical microcircuits for predictive coding. Neuron 76:695–711 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bear MF, Carnes KM, Ebner FF. 1985. An investigation of cholinergic circuitry in cat striate cortex using acetylcholinesterase histochemistry. J. Comp. Neurol 234:411–30 [DOI] [PubMed] [Google Scholar]
- Beierlein M, Gibson JR, Connors BW. 2003. Two dynamically distinct inhibitory networks in layer 4 of the neocortex. J. Neurophysiol 90:2987–3000 [DOI] [PubMed] [Google Scholar]
- Benevento LA, Ebner FF. 1971. The areas and layers of corticocortical terminations in the visual cortex of the Virginia opossum. J. Comp. Neurol 141:157–89 [DOI] [PubMed] [Google Scholar]
- Berger B, Tassin JP, Blanc G, Moyne MA, Thierry AM. 1974. Histochemical confirmation for dopaminergic innervation of the rat cerebral cortex after destruction of the noradrenergic ascending pathways. Brain Res. 81:332–37 [DOI] [PubMed] [Google Scholar]
- Berger B, Trottier S, Verney C, Gaspar P, Alvarez C. 1988. Regional and laminar distribution of the dopamine and serotonin innervation in the macaque cerebral cortex: a radioautographic study. J. Comp. Neurol 273:99–119 [DOI] [PubMed] [Google Scholar]
- Björklund A, Divac I, Lindvall O. 1978. Regional distribution of catecholamines in monkey cerebral cortex, evidence for a dopaminergic innervation of the primate prefrontal cortex. Neurosci. Lett 7:115–19 [DOI] [PubMed] [Google Scholar]
- Boldog E, Bakken TE, Hodge RD, Novotny M, Aevermann BD, et al. 2018. Transcriptomic and morphophysiological evidence for a specialized human cortical GABAergic cell type. Nat. Neurosci 21:1185–95 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bolkan SS, Stujenske JM, Parnaudeau S, Spellman TJ, Rauffenbart C, et al. 2017. Thalamic projections sustain prefrontal activity during working memory maintenance. Nat. Neurosci 20:987–96 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Callaway EM. 2004. Feedforward, feedback and inhibitory connections in primate visual cortex. Neural. Netw 17:625–32 [DOI] [PubMed] [Google Scholar]
- Campbell MJ, Lewis DA, Foote SL, Morrison JH. 1987. Distribution of choline acetyltransferase-, serotonin-, dopamine-β-hydroxylase-, tyrosine hydroxylase-immunoreactive fibers in monkey primary auditory cortex. J. Comp. Neurol 261:209–20 [DOI] [PubMed] [Google Scholar]
- Cappe C, Barone P. 2005. Heteromodal connections supporting multisensory integration at low levels of cortical processing in the monkey. Eur. J. Neurosci 22:2886–902 [DOI] [PubMed] [Google Scholar]
- Cauller L. 1995. Layer I of primary sensory neocortex: where top-down converges upon bottom-up. Behav. Brain Res 71:163–70 [DOI] [PubMed] [Google Scholar]
- Cauller LJ, Clancy B, Connors BW. 1998. Backward cortical projections to primary somatosensory cortex in rats extend long horizontal axons in layer I. J. Comp. Neurol 390:297–310 [PubMed] [Google Scholar]
- Cauller LJ, Connors BW. 1994. Synaptic physiology of horizontal afferents to layer I in slices of rat SI neocortex. J. Neurosci 14:751–62 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cauller LJ, Kulics AT. 1988. A comparison of awake and sleeping cortical states by analysis of the somatosensory-evoked response of postcentral area 1 in rhesus monkey. Exp. Brain Res 72:584–92 [DOI] [PubMed] [Google Scholar]
- Cauller LJ, Kulics AT. 1991. The neural basis of the behaviorally relevant N1 component of the somatosensory-evoked potential in SI cortex of awake monkeys: evidence that backward cortical projections signal conscious touch sensation. Exp. Brain Res 84:607–19 [DOI] [PubMed] [Google Scholar]
- Chen Z, Wimmer RD, Wilson MA, Halassa MM. 2015. Thalamic circuit mechanisms link sensory processing in sleep and attention. Front. Neural. Circuits 9:83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chittajallu R, Pelkey KA, McBain CJ. 2013. Neurogliaform cells dynamically regulate somatosensory integration via synapse-specific modulation. Nat. Neurosci 16:13–15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chou XL, Fang Q, Yan L, Zhong W, Peng B, et al. 2020. Contextual and cross-modality modulation of auditory cortical processing through pulvinar mediated suppression. eLife 9:e54157 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chu Z, Galarreta M, Hestrin S. 2003. Synaptic interactions of late-spiking neocortical neurons in layer 1. J. Neurosci 23:96–102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cichon J, Gan WB. 2015. Branch-specific dendritic Ca2+ spikes cause persistent synaptic plasticity. Nature 520:180–85 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clasca F, Rubio-Garrido P, Jabaudon D. 2012. Unveiling the diversity of thalamocortical neuron subtypes. Eur. J. Neurosci 35:1524–32 [DOI] [PubMed] [Google Scholar]
- Clavagnier S, Falchier A, Kennedy H. 2004. Long-distance feedback projections to area V1: implications for multisensory integration, spatial awareness, and visual consciousness. Cogn. Affect. Behav. Neurosci 4:117–26 [DOI] [PubMed] [Google Scholar]
- Coogan TA, Burkhalter A. 1993. Hierarchical organization of areas in rat visual cortex. J. Neurosci 13:3749–72 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cruikshank SJ, Ahmed OJ, Stevens TR, Patrick SL, Gonzalez AN, et al. 2012. Thalamic control of layer 1 circuits in prefrontal cortex. J. Neurosci 32:17813–23 [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Lima AD, Bloom FE, Morrison JH. 1988. Synaptic organization of serotonin-immunoreactive fibers in primary visual cortex of the macaque monkey. J. Comp. Neurol 274:280–94 [DOI] [PubMed] [Google Scholar]
- DeFelipe J, Marco P, Busturia I, Merchan-Perez A. 1999. Estimation of the number of synapses in the cerebral cortex: methodological considerations. Cereb. Cortex 9:722–32 [DOI] [PubMed] [Google Scholar]
- Descarries L, Lemay B, Doucet G, Berger B. 1987. Regional and laminar density of the dopamine innervation in adult rat cerebral cortex. Neuroscience 21:807–24 [DOI] [PubMed] [Google Scholar]
- Desimone R, Duncan J. 1995. Neural mechanisms of selective visual attention. Annu. Rev. Neurosci 18:193–222 [DOI] [PubMed] [Google Scholar]
- Doron G, Shin JN, Takahashi N, Bocklisch C, Skenderi S, et al. 2020. Perirhinal input to neocortical layer 1 controls learning. Science 370:eaaz3136 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Douglas RJ, Martin KA. 1991. A functional microcircuit for cat visual cortex. J. Physiol 440:735–69 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Douglas RJ, Martin KA. 2004. Neuronal circuits of the neocortex. Annu. Rev. Neurosci 27:419–51 [DOI] [PubMed] [Google Scholar]
- D’Souza RD, Burkhalter A. 2017. A laminar organization for selective cortico-cortical communication. Front. Neuroanat 11:71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Emson PC, Koob GF. 1978. The origin and distribution of dopamine-containing afferents to the rat frontal cortex. Brain Res. 142:249–67 [DOI] [PubMed] [Google Scholar]
- Felleman DJ, Van Essen DC. 1991. Distributed hierarchical processing in the primate cerebral cortex. Cereb. Cortex 1:1–47 [DOI] [PubMed] [Google Scholar]
- Fiser A, Mahringer D, Oyibo HK, Petersen AV, Leinweber M, Keller GB. 2016. Experience-dependent spatial expectations in mouse visual cortex. Nat. Neurosci 19:1658–64 [DOI] [PubMed] [Google Scholar]
- Fitzpatrick KA, Imig TJ. 1980. Auditory cortico-cortical connections in the owl monkey. J. Comp. Neurol 192:589–610 [DOI] [PubMed] [Google Scholar]
- Frick A, Johnston D. 2005. Plasticity of dendritic excitability. J. Neurobiol 64:100–15 [DOI] [PubMed] [Google Scholar]
- Friedman DP. 1983. Laminar patterns of termination of cortico-cortical afferents in the somatosensory system. Brain Res. 273:147–51 [DOI] [PubMed] [Google Scholar]
- Friedman DP, Murray EA, O’Neill JB, Mishkin M. 1986. Cortical connections of the somatosensory fields of the lateral sulcus of macaques: evidence for a corticolimbic pathway for touch. J. Comp. Neurol 252:323–47 [DOI] [PubMed] [Google Scholar]
- Friston K. 2018. Does predictive coding have a future? Nat. Neurosci 21:1019–21 [DOI] [PubMed] [Google Scholar]
- Fuxe K, Hamberger B, Hokfelt T. 1968. Distribution of noradrenaline nerve terminals in cortical areas of the rat. Brain Res. 8:125–31 [DOI] [PubMed] [Google Scholar]
- Gambino F, Pages S, Kehayas V, Baptista D, Tatti R, et al. 2014. Sensory-evoked LTP driven by dendritic plateau potentials in vivo. Nature 515:116–19 [DOI] [PubMed] [Google Scholar]
- Gao C, Leng Y, Ma J, Rooke V, Rodriguez-Gonzalez S, et al. 2020. Two genetically, anatomically and functionally distinct cell types segregate across anteroposterior axis of paraventricular thalamus. Nat. Neurosci 23:217–28 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gharaei S, Honnuraiah S, Arabzadeh E, Stuart GJ. 2020. Superior colliculus modulates cortical coding of somatosensory information. Nat. Commun 11:1693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilbert CD, Li W. 2013. Top-down influences on visual processing. Nat. Rev. Neurosci 14:350–63 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilbert CD, Sigman M. 2007. Brain states: top-down influences in sensory processing. Neuron 54:677–96 [DOI] [PubMed] [Google Scholar]
- Golding NL, Staff NP, Spruston N. 2002. Dendritic spikes as a mechanism for cooperative long-term potentiation. Nature 418:326–31 [DOI] [PubMed] [Google Scholar]
- Guillery RW. 1995. Anatomical evidence concerning the role of the thalamus in corticocortical communication: a brief review. J. Anat 187(Pt. 3):583–92 [PMC free article] [PubMed] [Google Scholar]
- Guo ZV, Inagaki HK, Daie K, Druckmann S, Gerfen CR, Svoboda K. 2017. Maintenance of persistent activity in a frontal thalamocortical loop. Nature 545:181–86 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halassa MM, Kastner S. 2017. Thalamic functions in distributed cognitive control. Nat. Neurosci 20:1669–79 [DOI] [PubMed] [Google Scholar]
- Halassa MM, Sherman SM. 2019. Thalamocortical circuit motifs: a general framework. Neuron 103:762–70 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harnett MT, Xu NL, Magee JC, Williams SR. 2013. Potassium channels control the interaction between active dendritic integration compartments in layer 5 cortical pyramidal neurons. Neuron 79:516–29 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris JA, Mihalas S, Hirokawa KE, Whitesell JD, Choi H, et al. 2019. Hierarchical organization of cortical and thalamic connectivity. Nature 575:195–202 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Häusser M, Spruston N, Stuart GJ. 2000. Diversity and dynamics of dendritic signaling. Science 290:739–44 [DOI] [PubMed] [Google Scholar]
- He M, Tucciarone J, Lee S, Nigro MJ, Kim Y, et al. 2016. Strategies and tools for combinatorial targeting of GABAergic neurons in mouse cerebral cortex. Neuron 91:1228–43 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heeger DJ. 2017. Theory of cortical function. PNAS 114:1773–82 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herkenham M. 1979. The afferent and efferent connections of the ventromedial thalamic nucleus in the rat. J. Comp. Neurol 183:487–517 [DOI] [PubMed] [Google Scholar]
- Hestrin S, Armstrong WE. 1996. Morphology and physiology of cortical neurons in layer I. J. Neurosci 16:5290–300 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Honjoh S, Sasai S, Schiereck SS, Nagai H, Tononi G, Cirelli C. 2018. Regulation of cortical activity and arousal by the matrix cells of the ventromedial thalamic nucleus. Nat. Commun 9:2100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hubel DH. 1982. Cortical neurobiology: a slanted historical perspective. Annu. Rev. Neurosci 5:363–70 [DOI] [PubMed] [Google Scholar]
- Ibrahim LA, Huang S, Fernandez-Otero M, Sherer M, Vemuri S, et al. 2021. Bottom-up inputs are required for the establishment of top-down connectivity onto cortical layer 1 neurogliaform cells. bioRxiv 2021.01.08.425944. 10.1101/2021.01.08.425944 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ibrahim LA, Mesik L, Ji XY, Fang Q, Li HF, et al. 2016. Cross-modality sharpening of visual cortical processing through layer-1-mediated inhibition and disinhibition. Neuron 89:1031–45 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jarsky T, Roxin A, Kath WL, Spruston N. 2005. Conditional dendritic spike propagation following distal synaptic activation of hippocampal CA1 pyramidal neurons. Nat. Neurosci 8:1667–76 [DOI] [PubMed] [Google Scholar]
- Jiang X, Shen S, Cadwell CR, Berens P, Sinz F, et al. 2015. Principles of connectivity among morphologically defined cell types in adult neocortex. Science 350:aac9462 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang X, Wang G, Lee AJ, Stornetta RL, Zhu JJ. 2013. The organization of two new cortical interneuronal circuits. Nat. Neurosci 16:210–18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones EG. 2001. The thalamic matrix and thalamocortical synchrony. Trends Neurosci. 24:595–601 [DOI] [PubMed] [Google Scholar]
- Jones EG. 2007. The Thalamus. Cambridge, UK: Cambridge Univ. Press [Google Scholar]
- Jones EG. 2009. Synchrony in the interconnected circuitry of the thalamus and cerebral cortex. Ann. N. Y. Acad. Sci 1157:10–23 [DOI] [PubMed] [Google Scholar]
- Juavinett AL, Callaway EM. 2015. Pattern and component motion responses in mouse visual cortical areas. Curr. Biol 25:1759–64 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalmbach A, Hedrick T, Waters J. 2012. Selective optogenetic stimulation of cholinergic axons in neocortex. J. Neurophysiol 107:2008–19 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kapfer C, Glickfeld LL, Atallah BV, Scanziani M. 2007. Supralinear increase of recurrent inhibition during sparse activity in the somatosensory cortex. Nat. Neurosci 10:743–53 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kastner S, Ungerleider LG. 2000. Mechanisms of visual attention in the human cortex. Annu. Rev. Neurosci 23:315–41 [DOI] [PubMed] [Google Scholar]
- Kayser C, Petkov CI, Augath M, Logothetis NK. 2005. Integration of touch and sound in auditory cortex. Neuron 48:373–84 [DOI] [PubMed] [Google Scholar]
- Keller AJ, Roth MM, Scanziani M. 2020. Feedback generates a second receptive field in neurons of the visual cortex. Nature 582:545–49 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keller GB, Bonhoeffer T, Hubener M. 2012. Sensorimotor mismatch signals in primary visual cortex of the behaving mouse. Neuron 74:809–15 [DOI] [PubMed] [Google Scholar]
- Keller GB, Mrsic-Flogel TD. 2018. Predictive processing: a canonical cortical computation. Neuron 100:424–35 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kerlin A, Mohar B, Flickinger D, MacLennan BJ, Dean MB, et al. 2019. Functional clustering of dendritic activity during decision-making. eLife 8:e46966 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Killackey H, Ebner F. 1973. Convergent projection of three separate thalamic nuclei on to a single cortical area. Science 179:283–85 [DOI] [PubMed] [Google Scholar]
- Kim EJ, Juavinett AL, Kyubwa EM, Jacobs MW, Callaway EM. 2015. Three types of cortical layer 5 neurons that differ in brain-wide connectivity and function. Neuron 88:1253–67 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kinomura S, Larsson J, Gulyas B, Roland PE. 1996. Activation by attention of the human reticular formation and thalamic intralaminar nuclei. Science 271:512–15 [DOI] [PubMed] [Google Scholar]
- Kosofsky BE, Molliver ME, Morrison JH, Foote SL. 1984. The serotonin and norepinephrine innervation of primary visual cortex in the cynomolgus monkey (Macaca fascicularis). J. Comp. Neurol 230:168–78 [DOI] [PubMed] [Google Scholar]
- Krnjevic K, Silver A. 1965. A histochemical study of cholinergic fibres in the cerebral cortex. J. Anat 99:711–59 [PMC free article] [PubMed] [Google Scholar]
- Kubota Y, Shigematsu N, Karube F, Sekigawa A, Kato S, et al. 2011. Selective coexpression of multiple chemical markers defines discrete populations of neocortical GABAergic neurons. Cereb. Cortex 21:1803–17 [DOI] [PubMed] [Google Scholar]
- Kuramoto E, Furuta T, Nakamura KC, Unzai T, Hioki H, Kaneko T. 2009. Two types of thalamocortical projections from the motor thalamic nuclei of the rat: a single neuron-tracing study using viral vectors. Cereb. Cortex 19:2065–77 [DOI] [PubMed] [Google Scholar]
- Kuramoto E, Pan S, Furuta T, Tanaka YR, Iwai H, et al. 2017. Individual mediodorsal thalamic neurons project to multiple areas of the rat prefrontal cortex: a single neuron-tracing study using virus vectors. J. Comp. Neurol 525:166–85 [DOI] [PubMed] [Google Scholar]
- Kuypers HG, Szwarcbart MK, Mishkin M, Rosvold HE. 1965. Occipitotemporal corticocortical connections in the rhesus monkey. Exp. Neurol 11:245–62 [DOI] [PubMed] [Google Scholar]
- Lacefield CO, Pnevmatikakis EA, Paninski L, Bruno RM. 2019. Reinforcement learning recruits somata and apical dendrites across layers of primary sensory cortex. Cell Rep. 26:2000–8.e2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larkum M. 2013. A cellular mechanism for cortical associations: an organizing principle for the cerebral cortex. Trends Neurosci. 36:141–51 [DOI] [PubMed] [Google Scholar]
- Larkum ME, Nevian T, Sandler M, Polsky A, Schiller J. 2009. Synaptic integration in tuft dendrites of layer 5 pyramidal neurons: a new unifying principle. Science 325:756–60 [DOI] [PubMed] [Google Scholar]
- Larkum ME, Zhu JJ, Sakmann B. 1999. A new cellular mechanism for coupling inputs arriving at different cortical layers. Nature 398:338–41 [DOI] [PubMed] [Google Scholar]
- Larkum ME, Zhu JJ, Sakmann B. 2001. Dendritic mechanisms underlying the coupling of the dendritic with the axonal action potential initiation zone of adult rat layer 5 pyramidal neurons. J. Physiol 533:447–66 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee AJ, Wang G, Jiang X, Johnson SM, Hoang ET, et al. 2015. Canonical organization of layer 1 neuron-led cortical inhibitory and disinhibitory interneuronal circuits. Cereb. Cortex 25:2114–26 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee S, Kruglikov I, Huang ZJ, Fishell G, Rudy B. 2013. A disinhibitory circuit mediates motor integration in the somatosensory cortex. Nat. Neurosci 16:1662–70 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leinweber M, Ward DR, Sobczak JM, Attinger A, Keller GB. 2017. A sensorimotor circuit in mouse cortex for visual flow predictions. Neuron 95:1420–32.e5 [DOI] [PubMed] [Google Scholar]
- Letzkus JJ, Kampa BM, Stuart GJ. 2006. Learning rules for spike timing–dependent plasticity depend on dendritic synapse location. J. Neurosci 26:10420–29 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Letzkus JJ, Wolff SB, Meyer EM, Tovote P, Courtin J, et al. 2011. A disinhibitory microcircuit for associative fear learning in the auditory cortex. Nature 480:331–35 [DOI] [PubMed] [Google Scholar]
- Levitt P, Moore RY. 1978. Noradrenaline neuron innervation of the neocortex in the rat. Brain Res. 139:219–31 [DOI] [PubMed] [Google Scholar]
- Lewis DA. 1991. Distribution of choline acetyltransferase-immunoreactive axons in monkey frontal cortex. Neuroscience 40:363–74 [DOI] [PubMed] [Google Scholar]
- Lewis DA, Campbell MJ, Foote SL, Goldstein M, Morrison JH. 1987. The distribution of tyrosine hydroxylase-immunoreactive fibers in primate neocortex is widespread but regionally specific. J. Neurosci 7:279–90 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Llinas R, Ribary U. 2001. Consciousness and the brain. The thalamocortical dialogue in health and disease. Ann. N. Y. Acad. Sci 929:166–75 [PubMed] [Google Scholar]
- Lorente de Nó R. 1938. The cerebral cortex: architecture, intracortical connections and motor projections. In Physiology of the Nervous System, ed. Fulton J, pp. 291–339. London: Oxford Med. Publ. [Google Scholar]
- Losonczy A, Makara JK, Magee JC. 2008. Compartmentalized dendritic plasticity and input feature storage in neurons. Nature 452:436–41 [DOI] [PubMed] [Google Scholar]
- Lysakowski A, Wainer BH, Bruce G, Hersh LB. 1989. An atlas of the regional and laminar distribution of choline acetyltransferase immunoreactivity in rat cerebral cortex. Neuroscience 28:291–336 [DOI] [PubMed] [Google Scholar]
- Magee JC. 2000. Dendritic integration of excitatory synaptic input. Nat. Rev. Neurosci 1:181–90 [DOI] [PubMed] [Google Scholar]
- Magee JC, Johnston D. 1997. A synaptically controlled, associative signal for Hebbian plasticity in hippocampal neurons. Science 275:209–13 [DOI] [PubMed] [Google Scholar]
- Mago A, Weber JP, Ujfalussy BB, Makara JK. 2020. Synaptic plasticity depends on the fine-scale input pattern in thin dendrites of CA1 pyramidal neurons. J. Neurosci 40:2593–605 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Major G, Larkum ME, Schiller J. 2013. Active properties of neocortical pyramidal neuron dendrites. Annu. Rev. Neurosci 36:1–24 [DOI] [PubMed] [Google Scholar]
- Manita S, Suzuki T, Homma C, Matsumoto T, Odagawa M, et al. 2015. A top-down cortical circuit for accurate sensory perception. Neuron 86:1304–16 [DOI] [PubMed] [Google Scholar]
- Marin-Padilla M. 1998. Cajal-Retzius cells and the development of the neocortex. Trends Neurosci. 21:64–71 [DOI] [PubMed] [Google Scholar]
- Marques T, Nguyen J, Fioreze G, Petreanu L. 2018. The functional organization of cortical feedback inputs to primary visual cortex. Nat. Neurosci 21:757–64 [DOI] [PubMed] [Google Scholar]
- Mashour GA. 2014. Top-down mechanisms of anesthetic-induced unconsciousness. Front. Syst. Neurosci 8:115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maunsell JH, van Essen DC. 1983. The connections of the middle temporal visual area (MT) and their relationship to a cortical hierarchy in the macaque monkey. J. Neurosci 3:2563–86 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mease RA, Metz M, Groh A. 2016. Cortical sensory responses are enhanced by the higher-order thalamus. Cell Rep. 14:208–15 [DOI] [PubMed] [Google Scholar]
- Mechawar N, Cozzari C, Descarries L. 2000. Cholinergic innervation in adult rat cerebral cortex: a quantitative immunocytochemical description. J. Comp. Neurol 428:305–18 [DOI] [PubMed] [Google Scholar]
- Melzer S, Monyer H. 2020. Diversity and function of corticopetal and corticofugal GABAergic projection neurons. Nat. Rev. Neurosci 21:499–515 [DOI] [PubMed] [Google Scholar]
- Meyer HS, Schwarz D, Wimmer VC, Schmitt AC, Kerr JN, et al. 2011. Inhibitory interneurons in a cortical column form hot zones of inhibition in layers 2 and 5A. PNAS 108:16807–12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller MW, Vogt BA. 1984. Direct connections of rat visual cortex with sensory, motor, and association cortices. J. Comp. Neurol 226:184–202 [DOI] [PubMed] [Google Scholar]
- Mitchell BD, Cauller LJ. 2001. Corticocortical and thalamocortical projections to layer I of the frontal neocortex in rats. Brain Res. 921:68–77 [DOI] [PubMed] [Google Scholar]
- Morrison JH, Foote SL, Molliver ME, Bloom FE, Lidov HG. 1982a. Noradrenergic and serotonergic fibers innervate complementary layers in monkey primary visual cortex: an immunohistochemical study. PNAS 79:2401–5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morrison JH, Foote SL, O’Connor D, Bloom FE. 1982b. Laminar, tangential and regional organization of the noradrenergic innervation of monkey cortex: dopamine-β-hydroxylase immunohistochemistry. Brain Res. Bull 9:309–19 [DOI] [PubMed] [Google Scholar]
- Morrissette AE, Chen PH, Bhamani C, Borden PY, Waiblinger C, et al. 2019. Unilateral optogenetic inhibition and excitation of basal ganglia output affect directional lick choices and movement initiation in mice. Neuroscience 423:55–65 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muñoz W, Tremblay R, Levenstein D, Rudy B. 2017. Layer-specific modulation of neocortical dendritic inhibition during active wakefulness. Science 355:954–59 [DOI] [PubMed] [Google Scholar]
- Murayama M, Perez-Garci E, Nevian T, Bock T, Senn W, Larkum ME. 2009. Dendritic encoding of sensory stimuli controlled by deep cortical interneurons. Nature 457:1137–41 [DOI] [PubMed] [Google Scholar]
- Ohno S, Kuramoto E, Furuta T, Hioki H, Tanaka YR, et al. 2012. A morphological analysis of thalamocortical axon fibers of rat posterior thalamic nuclei: a single neuron tracing study with viral vectors. Cereb. Cortex 22:2840–57 [DOI] [PubMed] [Google Scholar]
- Oláh S, Fule M, Komlosi G, Varga C, Baldi R, et al. 2009. Regulation of cortical microcircuits by unitary GABA-mediated volume transmission. Nature 461:1278–81 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oswald AM, Doiron B, Rinzel J, Reyes AD. 2009. Spatial profile and differential recruitment of GABAB modulate oscillatory activity in auditory cortex. J. Neurosci 29:10321–34 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Overstreet-Wadiche L, McBain CJ. 2015. Neurogliaform cells in cortical circuits. Nat. Rev. Neurosci 16:458–68 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palmer LM, Schulz JM, Murphy SC, Ledergerber D, Murayama M, Larkum ME. 2012. The cellular basis of GABAB-mediated interhemispheric inhibition. Science 335:989–93 [DOI] [PubMed] [Google Scholar]
- Palmer LM, Shai AS, Reeve JE, Anderson HL, Paulsen O, Larkum ME. 2014. NMDA spikes enhance action potential generation during sensory input. Nat. Neurosci 17:383–90 [DOI] [PubMed] [Google Scholar]
- Pandya DN, Dye P, Butters N. 1971. Efferent cortico-cortical projections of the prefrontal cortex in the rhesus monkey. Brain Res. 31:35–46 [DOI] [PubMed] [Google Scholar]
- Pandya DN, Kuypers HG. 1969. Cortico-cortical connections in the rhesus monkey. Brain Res. 13:13–36 [DOI] [PubMed] [Google Scholar]
- Pandya DN, Rosene DL. 1993. Laminar termination patterns of thalamic, callosal, and association afferents in the primary auditory area of the rhesus monkey. Exp. Neurol 119:220–34 [DOI] [PubMed] [Google Scholar]
- Pandya DN, Sanides F. 1973. Architectonic parcellation of the temporal operculum in rhesus monkey and its projection pattern. Z. Anat. Entwicklungsgesch 139:127–61 [DOI] [PubMed] [Google Scholar]
- Petreanu L, Mao T, Sternson SM, Svoboda K. 2009. The subcellular organization of neocortical excitatory connections. Nature 457:1142–45 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfeffer CK, Xue M, He M, Huang ZJ, Scanziani M. 2013. Inhibition of inhibition in visual cortex: the logic of connections between molecularly distinct interneurons. Nat. Neurosci 16:1068–76 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Polsky A, Mel BW, Schiller J. 2004. Computational subunits in thin dendrites of pyramidal cells. Nat. Neurosci 7:621–27 [DOI] [PubMed] [Google Scholar]
- Price CJ, Scott R, Rusakov DA, Capogna M. 2008. GABAB receptor modulation of feedforward inhibition through hippocampal neurogliaform cells. J. Neurosci 28:6974–82 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pronneke A, Scheuer B, Wagener RJ, Mock M, Witte M, Staiger JF. 2015. Characterizing VIP neurons in the barrel cortex of VIPcre/tdTomato mice reveals layer-specific differences. Cereb. Cortex 25:4854–68 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Purushothaman G, Marion R, Li K, Casagrande VA. 2012. Gating and control of primary visual cortex by pulvinar. Nat. Neurosci 15:905–12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rao RP, Ballard DH. 1999. Predictive coding in the visual cortex: a functional interpretation of some extra-classical receptive-field effects. Nat. Neurosci 2:79–87 [DOI] [PubMed] [Google Scholar]
- Rockland KS, Ojima H. 2003. Multisensory convergence in calcarine visual areas in macaque monkey. Int. J. Psychophysiol 50:19–26 [DOI] [PubMed] [Google Scholar]
- Rockland KS, Pandya DN. 1979. Laminar origins and terminations of cortical connections of the occipital lobe in the rhesus monkey. Brain Res. 179:3–20 [DOI] [PubMed] [Google Scholar]
- Roth MM, Dahmen JC, Muir DR, Imhof F, Martini FJ, Hofer SB. 2016. Thalamic nuclei convey diverse contextual information to layer 1 of visual cortex. Nat. Neurosci 19:299–307 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubio-Garrido P, Perez-de-Manzo F, Porrero C, Galazo MJ, Clasca F. 2009. Thalamic input to distal apical dendrites in neocortical layer 1 is massive and highly convergent. Cereb. Cortex 19:2380–95 [DOI] [PubMed] [Google Scholar]
- Saalmann YB, Pinsk MA, Wang L, Li X, Kastner S. 2012. The pulvinar regulates information transmission between cortical areas based on attention demands. Science 337:753–56 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scannell JW, Blakemore C, Young MP. 1995. Analysis of connectivity in the cat cerebral cortex. J. Neurosci 15:1463–83 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmitt LI, Wimmer RD, Nakajima M, Happ M, Mofakham S, Halassa MM. 2017. Thalamic amplification of cortical connectivity sustains attentional control. Nature 545:219–23 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schneider DM, Mooney R. 2018. How movement modulates hearing. Annu. Rev. Neurosci 41:553–72 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schneider DM, Nelson A, Mooney R. 2014. A synaptic and circuit basis for corollary discharge in the auditory cortex. Nature 513:189–94 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schuman B, Machold RP, Hashikawa Y, Fuzik J, Fishell GJ, Rudy B. 2019. Four unique interneuron populations reside in neocortical layer 1. J. Neurosci 39:125–39 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwiedrzik CM, Freiwald WA. 2017. High-level prediction signals in a low-level area of the macaque face-processing hierarchy. Neuron 96:89–97.e4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sherman SM, Guillery RW. 2005. Exploring the Thalamus and Its Role in Cortical Function. Cambridge, MA: MIT Press [Google Scholar]
- Shipp S, Zeki S. 1989. The organization of connections between areas V5 and V2 in macaque monkey visual cortex. Eur. J. Neurosci 1:333–54 [DOI] [PubMed] [Google Scholar]
- Silberberg G, Markram H. 2007. Disynaptic inhibition between neocortical pyramidal cells mediated by Martinotti cells. Neuron 53:735–46 [DOI] [PubMed] [Google Scholar]
- Sjöström PJ, Häusser M. 2006. A cooperative switch determines the sign of synaptic plasticity in distal dendrites of neocortical pyramidal neurons. Neuron 51:227–38 [DOI] [PubMed] [Google Scholar]
- Spatz WB. 1977. Topographically organized reciprocal connections between areas 17 and MT (visual area of superior temporal sulcus) in the marmoset Callithrix jacchus. Exp. Brain Res 27:559–72 [DOI] [PubMed] [Google Scholar]
- Stanley J, Miall RC. 2007. Functional activation in parieto-premotor and visual areas dependent on congruency between hand movement and visual stimuli during motor-visual priming. Neuroimage 34:290–99 [DOI] [PubMed] [Google Scholar]
- Stehberg J, Dang PT, Frostig RD. 2014. Unimodal primary sensory cortices are directly connected by long-range horizontal projections in the rat sensory cortex. Front. Neuroanat 8:93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stepanyants A, Martinez LM, Ferecsko AS, Kisvarday ZF. 2009. The fractions of short- and long-range connections in the visual cortex. PNAS 106:3555–60 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steriade M. 1996. Awakening the brain. Nature 383:24–25 [DOI] [PubMed] [Google Scholar]
- Steriade M, Jones EG, Llinás RR 1990. Thalamic Oscillations and Signaling. New York: Wiley [Google Scholar]
- Stuart G, Spruston N. 1998. Determinants of voltage attenuation in neocortical pyramidal neuron dendrites. J. Neurosci 18:3501–10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stuart G, Spruston N, Sakmann B, Hausser M. 1997. Action potential initiation and backpropagation in neurons of the mammalian CNS. Trends Neurosci. 20:125–31 [DOI] [PubMed] [Google Scholar]
- Stuart GJ, Spruston N. 2015. Dendritic integration: 60 years of progress. Nat. Neurosci 18:1713–21 [DOI] [PubMed] [Google Scholar]
- Suzuki M, Larkum ME. 2020. General anesthesia decouples cortical pyramidal neurons. Cell 180:666–76.e13 [DOI] [PubMed] [Google Scholar]
- Takahashi H, Magee JC. 2009. Pathway interactions and synaptic plasticity in the dendritic tuft regions of CA1 pyramidal neurons. Neuron 62:102–11 [DOI] [PubMed] [Google Scholar]
- Takahashi N, Ebner C, Sigl-Glockner J, Moberg S, Nierwetberg S, Larkum ME. 2020. Active dendritic currents gate descending cortical outputs in perception. Nat. Neurosci 23:1277–85 [DOI] [PubMed] [Google Scholar]
- Takahashi N, Oertner TG, Hegemann P, Larkum ME. 2016. Active cortical dendrites modulate perception. Science 354:1587–90 [DOI] [PubMed] [Google Scholar]
- Tamas G, Lorincz A, Simon A, Szabadics J. 2003. Identified sources and targets of slow inhibition in the neocortex. Science 299:1902–5 [DOI] [PubMed] [Google Scholar]
- Tanaka YH, Tanaka YR, Kondo M, Terada SI, Kawaguchi Y, Matsuzaki M. 2018. Thalamocortical axonal activity in motor cortex exhibits layer-specific dynamics during motor learning. Neuron 100:244–58.e12 [DOI] [PubMed] [Google Scholar]
- Thierry AM, Blanc G, Sobel A, Stinus L, Glowinski J. 1973. Dopaminergic terminals in the rat cortex. Science 182:499–501 [DOI] [PubMed] [Google Scholar]
- Tigges J, Spatz WB, Tigges M. 1973. Reciprocal point-to-point connections between parastriate and striate cortex in the squirrel monkey (Saimiri). J. Comp. Neurol 148:481–89 [DOI] [PubMed] [Google Scholar]
- Tremblay R, Lee S, Rudy B. 2016. GABAergic interneurons in the neocortex: from cellular properties to circuits. Neuron 91:260–92 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Urban-Ciecko J, Fanselow EE, Barth AL. 2015. Neocortical somatostatin neurons reversibly silence excitatory transmission via GABAb receptors. Curr. Biol 25:722–31 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van der Werf YD, Witter MP, Groenewegen HJ. 2002. The intralaminar and midline nuclei of the thalamus. Anatomical and functional evidence for participation in processes of arousal and awareness. Brain Res. Rev 39:107–40 [DOI] [PubMed] [Google Scholar]
- Vicente R, Gollo LL, Mirasso CR, Fischer I, Pipa G. 2008. Dynamical relaying can yield zero time lag neuronal synchrony despite long conduction delays. PNAS 105:17157–62 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vogt BA, Pandya DN. 1987. Cingulate cortex of the rhesus monkey: II. Cortical afferents. J. Comp. Neurol 262:271–89 [DOI] [PubMed] [Google Scholar]
- Wang X, Tucciarone J, Jiang S, Yin F, Wang BS, et al. 2019. Genetic single neuron anatomy reveals fine granularity of cortical axo-axonic cells. Cell Rep. 26:3145–59.e5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weber JP, Andrasfalvy BK, Polito M, Mago A, Ujfalussy BB, Makara JK. 2016. Location-dependent synaptic plasticity rules by dendritic spine cooperativity. Nat. Commun 7:11380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams LE, Holtmaat A. 2019. Higher-order thalamocortical inputs gate synaptic long-term potentiation via disinhibition. Neuron 101:91–102.e4 [DOI] [PubMed] [Google Scholar]
- Williams SR, Stuart GJ. 2002. Dependence of EPSP efficacy on synapse location in neocortical pyramidal neurons. Science 295:1907–10 [DOI] [PubMed] [Google Scholar]
- Wilson MA, Molliver ME. 1991. The organization of serotonergic projections to cerebral cortex in primates: regional distribution of axon terminals. Neuroscience 44:537–53 [DOI] [PubMed] [Google Scholar]
- Wong-Riley M. 1979. Columnar cortico-cortical interconnections within the visual system of the squirrel and macaque monkeys. Brain Res. 162:201–17 [DOI] [PubMed] [Google Scholar]
- Wozny C, Williams SR. 2011. Specificity of synaptic connectivity between layer 1 inhibitory interneurons and layer 2/3 pyramidal neurons in the rat neocortex. Cereb. Cortex 21:1818–26 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu H, Jeong H-Y, Tremblay R, Rudy B. 2013. Neocortical somatostatin-expressing GABAergic interneurons disinhibit the thalamorecipient layer 4. Neuron 77:155–67 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu NL, Harnett MT, Williams SR, Huber D, O’Connor DH, et al. 2012. Nonlinear dendritic integration of sensory and motor input during an active sensing task. Nature 492:247–51 [DOI] [PubMed] [Google Scholar]
- Xu X, Roby KD, Callaway EM. 2010. Immunochemical characterization of inhibitory mouse cortical neurons: three chemically distinct classes of inhibitory cells. J. Comp. Neurol 518:389–404 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yeterian EH, Pandya DN, Tomaiuolo F, Petrides M. 2012. The cortical connectivity of the prefrontal cortex in the monkey brain. Cortex 48:58–81 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zagha E. 2020. Shaping the cortical landscape: functions and mechanisms of top-down cortical feedback pathways. Front. Syst. Neurosci 14:33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang S, Xu M, Kamigaki T, Hoang Do JP, Chang WC, et al. 2014. Long-range and local circuits for top-down modulation of visual cortex processing. Science 345:660–65 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang W, Bruno RM. 2019. High-order thalamic inputs to primary somatosensory cortex are stronger and longer lasting than cortical inputs. eLife 8:e44158 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou FM, Hablitz JJ. 1996. Morphological properties of intracellularly labeled layer I neurons in rat neocortex. J. Comp. Neurol 376:198–213 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.