Abstract
The indiscriminate use of chemical pesticides increasingly harms the health of living beings and the environment. Thus, biological control carried out by microorganisms has gained prominence, since it consists of an environmentally friendly alternative to the use of pesticides for controlling plant diseases. Herein, we evaluated the potential role of endophytic Trichoderma strains isolated from forest species of the Cerrado-Caatinga ecotone as biological control agents of crop pathogenic fungi. Nineteen Trichoderma strains were used to assess the antagonistic activity by in vitro bioassays against the plant pathogens Colletotrichum truncatum, Lasiodiplodia theobromae, Macrophomina phaseolina, and Sclerotium delphinii isolated from soybean, cacao, fava bean, and black pepper crops, respectively. All Trichoderma strains demonstrated inhibitory activity on pathogen mycelial growth, with maximum percent inhibition of 70% against C. truncatum, 78% against L. theobromae, 78% against M. phaseolina, and 69% against S. delphinii. Crude methanol extracts (0.5 to 2.0 mg mL-1) of Trichoderma strains were able to inhibit the growth of C. truncatum, except Trichoderma sp. T3 (UFPIT06) and T. orientale (UFPIT09 and UFPIT17) at 0.5 mg mL-1, indicating that the endophytes employ a biocontrol mechanism related to antibiosis, together with multiple mechanisms. Discriminant metabolites of Trichoderma extracts were unveiled by liquid chromatography-tandem mass spectrometry-based metabolomics combined with principal component analysis (PCA), which included antifungal metabolites and molecules with other bioactivities. These results highlight the biocontrol potential of Trichoderma strains isolated from the Cerrado-Caatinga ecotone against crop pathogenic fungi, providing support for ongoing research on disease control in agriculture.
Introduction
Diseases caused by fungi are among the most harmful to plants due to their rapid spread and the ability to adapt to various environmental conditions [1]. The most commonly used methods for the control of plant diseases caused by fungi include the use of chemical fungicides [1, 2], which have disadvantages such as the potential risk of soil and water contamination, damage to human health, and the development of resistance against fungicides by plant pathogens [2, 3].
Research on alternative methods of controlling fungal diseases has been widely developed, often requiring the integrated implementation of several methods, known as integrated disease management (IDM) [2, 4]. Among these methods are the use of disease-resistant cultivars, adequate water and soil management, fertilization, crop rotations, and biological control agents (BCA), aiming to maintain or increase agricultural production with a reduced application of chemical agents [5]. Many studies still need to be developed for the use of biological control of plant pathogens on a global scale, since biopesticides represent only approximately 2% of all pesticides sold in the world [6, 7].
A promising alternative method for plant pathogen control is based on the use of antagonistic microorganisms, such as endophytic fungi, capable of protecting their hosts from the action of pathogens [8, 9]. These microorganisms live inside plant tissues without causing damage in a complex mutualistic relationship, where endophytes receive nutrients and protection, while plants have advantages, such as greater resistance in environments with intense stress caused by biotic (insects, herbivores, nematodes, and phytopathogenic microorganisms) or abiotic factors (pH, temperature, drought and saline stresses, etc.) [10, 11].
Recent research has revealed that although endophytic microorganisms have received global interest, there are still several gaps in knowledge, such as the different biomes explored [12]. Considering that endophytes depend on host species and environmental conditions, the diversity of biomes and endemic plants found in Brazil represents a potential source of new beneficial microbial resources [13]. In this context, the Cerrado-Caatinga ecotone stands out [14], which corresponds to the transition area where ecological communities or ecosystems from the Cerrado and Caatinga biomes coincide [15].
The Cerrado and Caatinga biomes are recognized for their great importance. The Cerrado, also known as the Brazilian savanna, is one of the 25 biodiversity hotspots for conservation priorities in the world [16, 17], and the Caatinga is the only uniquely Brazilian biome, in which most of its biological heritage cannot be found anywhere else in the world [18]. The Cerrado-Caatinga ecotone occupies 1.3% of the Brazilian territory, extending over regions of the Piauí, Bahia, and Minas Gerais states [14], and it presents great species richness, whether from the biomes that formed them or endemic species [15]. Few studies have been carried out to explore the biocontrol potential of endophytic fungal biodiversity in this transition zone, which also requires attention due to increasing anthropogenic degradation with the expansion of agricultural production areas [19].
Trichoderma species have been tried as BCA and used as an alternative to synthetic pesticides to control a variety of plant diseases [20]. The biocontrol mechanisms of Trichoderma are based on the activation of multiple mechanisms, either indirectly, by competing for space and nutrients, promoting plant growth and plant defensive mechanisms, and antibiosis, or directly, by mycoparasitism [21, 22]. They are found in rhizospheric and non-rhizospheric soils, in addition to their endophytic relationships with many plants [22, 23]. Their biodiversity has been extensively investigated in various geographical locations, and their distribution varies with ecosystems [24, 25]. Therefore, it is fundamental to explore the biocontrol potential of Trichoderma strains isolated from native areas, since they represent a tool for sustainable food production.
In this study, we investigated the potential role of endophytic Trichoderma strains isolated from forest tree species of the Cerrado-Caatinga ecotone [26, 27] as biological control agents of crop pathogenic fungi. First, we evaluated the interaction between endophytes and pathogens by in vitro antagonism bioassays. The biocontrol factor related to antibiosis was examined using in vitro bioassays with crude methanolic extracts of Trichoderma strains and liquid chromatography-tandem mass spectrometry-based metabolomic approaches. Such data will support ongoing research to find new beneficial microbial resources to control plant diseases.
Material and methods
Strains and materials
The nineteen Trichoderma spp. isolates (S1 Table) were obtained from leaves of forest tree species (S2 Table), located in a Cerrado-Caatinga ecotone in Southwest Piauí, Brazil (8°51′7.48″ S and 44°11′39.95″ W) [26], and maintained in potato dextrose agar (PDA) culture medium (Himedia). This area comprised a fragment of one hectare within the legal reserve [26]. For the identification of the Trichoderma isolates, the gene regions for the translation elongation factor (tef1) and the second largest RNA polymerase subunit (rpb2) were amplified and sequenced, and the construction of phylogenetic trees was performed by comparing the sequences available in GenBank (National Center for Biotechnology Information, NCBI) [27].
The UFPIT01, UFPIT09, UFPIT12, UFPIT14, UFPIT15, UFPIT17, and UFPIT18 isolates were previously identified as T. orientale (Samuels & Petrini) Jaklitsch & Samuels; the UFPI02 isolate was previously identified as T. longibrachiatum Rifai; and the UFPI03, UFPI07, UFPI10, UFPI16, and UFPI19 isolates were previously identified as T. koningiopsis Samuels, Carm. Suárez & H.C. Evans [27]. The UFPIT04, UFPIT05, UFPIT06, UFPIT08, UFPIT11, and UFPIT13 isolates were not identified by comparing the sequences available in GenBank and may likely constitute new species; therefore, they were named Trichoderma sp. T1, Trichoderma sp. T2, Trichoderma sp. T3, Trichoderma sp. T4, Trichoderma sp. T5, and Trichoderma sp. T6, respectively [27]. The strains were registered in the National System for the Management of Genetic Heritage and Associated Traditional Knowledge (SisGen) by n° A7580C1 and A1B50F7, as recommended by the Brazilian Biodiversity Law (n° 13.123/15).
For plant pathogenic fungi tested for antagonism bioassays, Colletotrichum truncatum (Schwein.) Andrus & W.D. Moore strain was isolated from infected soybean pods, located in the same mesoregion where Trichoderma strains were found, through the cultivation of infected material in PDA medium incubated at 25°C under a 12 h photoperiod [28]. The Lasiodiplodia theobromae (Pat.) Griffon & Maubl. strain was isolated from cacao fruit with symptoms of Lasiodiplodia canker. Macrophomina phaseolina (Tassi) Goid. COUFPI 10 and COUFPI 11 strains were isolated from the seeds and roots of fava bean, respectively, placed in PDA medium and incubated at 25°C for seven days [29]. Sclerotium delphinii Welch COUFPI 209 and COUFPI 249 strains were isolated from black pepper with symptoms of concentric leaf spots by inoculating sclerotia in PDA medium [30]. All strains were maintained in PDA culture medium at 28°C in the absence of light and preserved using Castellani’s method.
Liquid chromatography–mass spectrometry (LC–MS)-grade methanol and acetonitrile were purchased from J.T. Baker (Center Valley, PA, USA). Analytical grade formic acid and sodium formate encephalin were purchased from J.T. Baker (Center Valley, PA, USA), and leucine enkephalin from Waters (Manchester, UK).
In vitro antagonism bioassays against plant pathogenic fungi
Culture medium fragments (1 cm2) with Trichoderma spp. mycelia and fungal plant pathogens (1 cm2), previously cultivated in PDA at 28°C, were transferred to PDA medium 5 cm apart from each other [31]. The plates were incubated at 28°C, and mycelial growth was evaluated daily for seven days. The experimental design was completely randomized with 20 treatments and 3 replications, totaling 60 experimental units for the bioassays of each plant pathogen. The treatments consisted of 19 Trichoderma isolates plus a control sample containing only the plant pathogen.
The antagonistic potential was measured as the percent inhibition, according to the formula: % inhibition = (DC-DT/DC)*100, where DT is the growth radius of the plant pathogen colony toward the antagonist and DC is the growth radius of the control [32]. The mycelial growth rate index (MGRI) was obtained from the averages of the daily values of mycelial growth for each treatment, according to the formula MGRI = Ʃ(D-Da)/N, where D = current average colony diameter, Da = average colony diameter from the previous day, and N = number of days after inoculation [33]. Analysis of variance followed by the Scott–Knott test at the 5% significance level was conducted using R v.3.5.2 software (R Core Team, Vienna, Austria).
Inhibitory activity bioassay of Trichoderma spp. organic extracts against C. truncatum
For the extraction of bioactive compounds, Trichoderma strains were inoculated on PDA at 28°C in the absence of light for four days. Subsequently, the culture media (60 x 15 mm) containing the fungal colonies were cut into small pieces, and cold methanol (15 mL) was added. The samples were vortexed for 1 min, allowed to rest for 5 min, and vortexed again for 1 min. Subsequently, the extracts were centrifuged at 4,000 g for 15 min at 4°C, and the supernatants were concentrated under a flow of nitrogen gas [34]. Then, the extracts were weighed, and dimethylsulfoxide (DMSO) was added to prepare a 100 mg mL-1 stock solution.
Methanolic extracts of Trichoderma spp. were used to evaluate the inhibitory activity against C. truncatum. For this purpose, fragments of the phytopathogen (9 mm2) were inoculated in the center of Petri dishes (60×15 mm) containing PDA with increasing concentrations of the extract (0.0, 0.5, 1.0, and 2.0 mg mL-1). For the control (0.0 mg mL-1), only DMSO was added. Plates were kept in B.O.D. incubator (Bio-Oxygen Demand) at 28°C in the absence of light, and colony diameters were measured daily for 10 days with the aid of a digital caliper. The experimental design was completely randomized with extracts from 19 isolates at concentrations of 0.0, 0.5, 1.0, and 2.0 mg L-1, with three replications for each concentration. Data were subjected to analysis of variance, followed by regression analysis using R software and SigmaPlot v11.0 (Systat Software Inc. Chicago, USA). Additionally, Pearson’s correlation analysis of the percent inhibition of Trichoderma strains against C. truncatum in co-culture and crude extract bioassays was performed.
Metabolic fingerprinting by liquid chromatography–high resolution mass spectrometry
Sample preparation
For extract preparation of the 19 Trichoderma isolates, the culture media (60 x 15 mm) containing the fungal colonies, previously cultivated on PDA at 28°C for four days, were cut into small pieces and extracted with methanol (15 mL), vortexed for 1 min, maintained at rest for 5 min, and vortexed again for 1 min. After filtration, the supernatants were concentrated to approximately 1 mL, lyophilized, and stored at -47°C until use. For analyses by ultra-high-performance liquid chromatography coupled with electrospray ionization quadrupole time-of-flight mass spectrometry (UPLC-ESI-Q-TOF-MS), lyophilized samples were reconstituted in a solution containing water/methanol/acetonitrile (1:2:2 v/v/v, 1 mL). The samples were vortexed for 1 min, sonicated for 30 min at room temperature, filtered using a 0.22 μm PTFE syringe filter (Millipore, USA), and transferred to vials for LC–MS analysis [35].
UHPLC-ESI-Q-TOF-MS analysis
An ACQUITY UPLC connected to a XEVO-G2XS QTOF mass spectrometer (Waters, Manchester, UK) equipped with an electrospray ion source was used. Liquid chromatography was performed using a Titan™ C18 UHPLC column (2.1 x 100 mm, 1.9 μm, Supelco). The column temperature was maintained at 45°C. The separation was performed at a flow rate of 0.4 mL min-1 under a gradient program in which the mobile phase consisted of (A) 0.1% formic acid (v/v) and (B) pure methanol. The gradient program was applied as follows (in % B): (t) = 0 min, 1%; t = 2.0 min, 1%; t = 8.0 min, 38%; t = 20 min, 99.5%; t = 25 min, 99.5%; t = 25.1 min, 1%; and t = 28 min, 1%, for a total analysis time of 28 minutes. The injection volume was 0.2 μL. Positive ion mode was recorded, and the instrument was operated in data-independent acquisition mode (MSE). The m/z range was 100–1700, with an acquisition rate of 0.5 sec per scan. The following instrumental parameters were used: capillary: 3.0 kV; cone: 40,000 V; desolvation temperature: 550°C; cone gas flow: 10 L h-1; desolvation gas flow: 900 L h-1. The collision energy was 20 to 60 eV for fragmentation. Leucine encephalin (molecular weight = 555.62; 200 pg μL-1 in 1:1 acetonitrile:water) was used as the lock mass for accurate mass measurements, and a 0.5 mM sodium formate solution was used for calibration. Samples were randomly analyzed.
Data processing and statistical data analysis
LC–MS raw data were processed using Progenesis QI 2.0 software (Nonlinear Dynamics, Newcastle, UK), which enabled the selection of possible adducts, peak alignment, deconvolution, and putative metabolite identification based on MSE experiments. Progenesis QI generates a table of the ions labeled according to their nominal masses and retention times as a function of their intensity for each sample. The MassBank database (https://massbank.eu) and Vaniya/Fiehn Natural Products Library (https://mona.fiehnlab.ucdavis.edu/) were used to perform the identification using the following search parameters: precursor mass error ≤ 5 ppm and fragment tolerance ≤ 10 ppm.
The list of extracted ion chromatograms by retention time was uploaded to the MetaboAnalyst 5.0 web platform (http://www.metaboanalyst.ca) for principal component analysis (PCA). Ions detected in at least 10% of the samples were retained for analysis, and an interquartile range (IQR) filter was used. Data were sum-normalized, and Pareto scaling was used. A heatmap and unsupervised hierarchical clustering were performed using 50 features with the lowest adjusted p value < 0.05 depicting differential peaks.
Results and discussion
Endophytic Trichoderma strains from forest species inhibit several crop pathogenic fungi
The antagonistic potential of the endophytic Trichoderma spp. strains was investigated against different plant pathogens. The 19 Trichoderma spp. isolates demonstrated inhibitory activity against mycelial growth, ranging from 50 to 70% for C. truncatum (Fig 1A), 30 to 78% for L. theobromae (Fig 1B), 49 to 78% for M. phaseolina COUFPI 10 (Fig 1C), 58 to 74% for M. phaseolina COUFPI 11, 6 to 62% for S. delphinii COUFPI 209 (Fig 1D), and 2 to 69% for S. delphinii COUFPI249.
Fig 1. In vitro percent inhibition of Trichoderma spp. isolates against C. truncatum (A), L. theobromae (B), M. phaseolina COUFPI 10 and COUFPI 11 (C), and S. delphinii COUFPI 209 and COUFPI 249 (D).
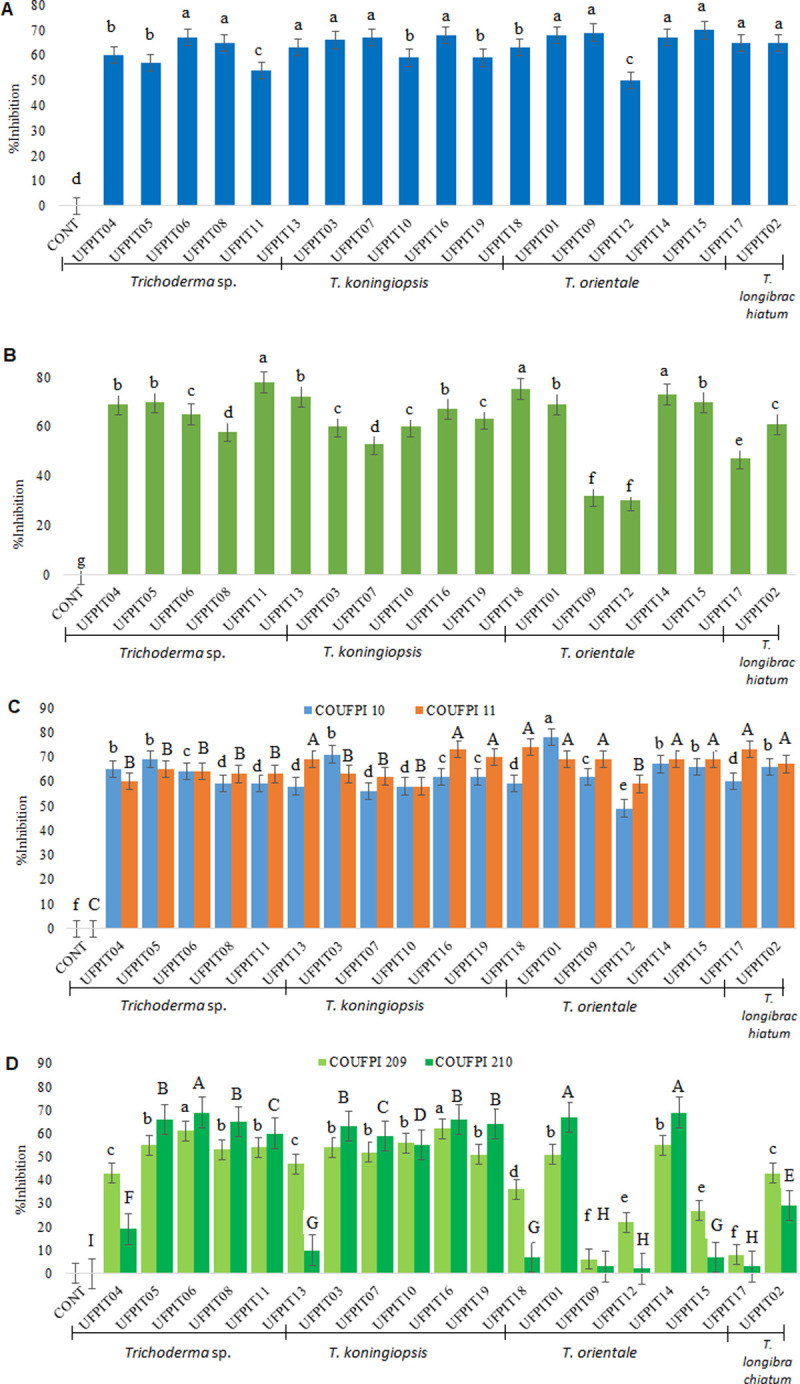
Averages followed by the same lowercase or capital letter are not significantly different by the Scott–Knott test at the 5% confidence level. The variation coefficients (CVs) were 4.67% for L. theobromae, 4.55% for M. phaseolina COUFPI 10, 5.96% for M. phaseolina COUFPI 11, 8.37% for S. delphinii COUFPI 209 and 6.02% for S. delphinii COUFPI 249.
Regarding the inhibition of C. truncatum (Fig 1A), the fungi T. orientale (UFPIT01, UFPIT09, UFPIT14, UFPIT15, and UFPIT17), T. longibrachiatum (UFPIT02), T. koningiopsis (UFPIT03, UFPIT07, and UFPIT16), Trichoderma sp. T3 (UFPIT06), Trichoderma sp. T4 (UFPIT08), and Trichoderma sp. T6 (UFPIT13) stood out with the highest percent inhibition, from 63 to 70%, and Trichoderma sp. T5 (UFPIT11) and T. orientale (UFPIT12), with the lowest values ranging from 50 to 54%. Trichoderma sp. T5 (UFPIT11) and T. orientale (UFPIT14 and UFPIT18) showed the highest percentage inhibition against L. theobromae (Fig 1B) of 73–78%, while T. orientale (UFPIT09 and UFPIT12) had the lowest performance of 30–32%.
Against M. phaseolina COUFPI 10 (Fig 1C), T. orientale UFPIT01 and UFPIT12 yielded the highest (78%) and lowest (49%) percent inhibition, respectively, while the other isolates showed intermediate values, above 50% inhibition. T. orientale (UFPIT01, UFPIT09, UFPIT14, UFPIT15, UFPIT17, UFPIT18), T. longibrachiatum (UFPIT02), Trichoderma sp. T6 (UFPIT13), and T. koningiopsis (UFPIT16 and UFPIT19) stood out in inhibiting the growth of M. phaseolina COUFPI 11, from 67 to 74%, and the others, with lower values, ranging from 58 to 66% inhibition, did not differ statistically.
The inhibitory activity against S. delphinii COUFPI 209 (Fig 1D) was highest in Trichoderma sp. T3 UFPIT06 (61%) and T. koningiopsis UFPIT16 (62%) and lowest in T. orientale UFPIT09 (6%) and T. orientale UFPIT17 (8%), the others ranged from 22 to 56%. In S. delphinii COUFPI 249 (Fig 1D), the fungi T. orientale (UFPIT01, UFPIT14 with 67% and 69%, respectively) and Trichoderma sp. T3 UFPIT06 (69%) presented the best results, with T. orientale (UFPIT09, UFPIT12, and UFPIT17 with 3, 2 and 3%, respectively) showing less effectiveness.
Most endophytes reduced the MGRI of the plant pathogen colonies, differing statistically from the control treatments (S1 Fig), except for S. delphinii COUFPI 249 paired with T. orientale UFPIT09. All isolates stood out in reducing the MGRI for C. truncatum (S1A Fig), with the highest indices observed for Trichoderma sp. T1 (UFPIT04), T. orientale (UFPIT12), Trichoderma sp. T6 (UFPIT13), and T. koningiopsis (UFPIT19) strains. Against L. theobromae, only the isolate T. orientale (UFPIT12) showed the highest MGRI (S1B Fig).
T. longibrachiatum (UFPIT02), T. koningiopsis (UFPIT03 and UFPIT10), Trichoderma sp. T2 (UFPIT05), Trichoderma sp. T3 (UFPIT06), T. orientale (UFPIT09, UFPIT14, UFPIT15, and UFPIT17), Trichoderma sp. T5 (UFPIT11), and Trichoderma sp. T6 (UFPIT13) stood out in reducing the MGRI of M. phaseolina, while T. koningiopsis (UFPIT03 and UFPIT16) stood out against M. phaseolina COUFPI 11 (S1C Fig). The MGRI of S. delphinii COUFPI 209 was reduced for all isolates, and the highest indices were obtained when paired with T. orientale (UFPIT09, UFPIT12, and UFPIT17), while for S. delphinii COUFPI 249, Trichoderma sp. T1 (UFPIT04) and Trichoderma sp. T6 (UFPIT13) showed the highest MGRI (S1D Fig).
Several studies have shown the efficacy of Trichoderma strains against C. truncatum. The species T. harzianum and T. asperellum showed percent inhibition of 75 and 73%, respectively, against this pathogen [36]. T. virens, T. longibrachiatum, and T. koningii also inhibited the growth of C. truncatum with %inhibition of 54 to 81% [37]. Commercial formulations based on T. viride, T. harzianum, and T. hamatum promoted %inhibition ranging from 67 to 81% [38]. In our work, similar results were obtained against this pathogen for the species T. orientale, T. longibrachiatum, T. koningiopsis and unidentified Trichoderma isolates.
The species T. harzianum, T. asperellum, T. atroviride, and T. virens showed percent inhibition in the range of 29 to 54% against L. theobromae [39], while T. koningii and T. viride reached 75 to 80% [40]. T. pseudokoningii, T. hamatum, T. koningii, and T. reesei also significantly inhibited pathogen growth by 62 to 90% [41]. These values corroborate the %inhibition observed in our study; however, to our knowledge, there are no reports of studies about the biocontrol potential of T. orientale, T. koningiopsis and T. longibrachiatum against L. theobromae.
In previous studies, T. longibrachiatum showed a percent inhibition of 58% against M. phaseolina [42], while T. koningiopsis strains ranging from 15 to 70% [43]. Similar results were obtained in our study for these species; however, there are no reports about T. orientale against M. phaseolina. Swain et al. (2021) investigated the biocontrol potential of T. erinaceum and T. hebeiensis against S. delphiii and found a percent inhibition of approximately 75%, which is the only study of growth inhibition of this pathogen using Trichoderma strains [44]. This is the first report that demonstrates the biocontrol potential of the species T. orientale, T. koningiopsis and T. longibrachiatum against S. delphiii.
The high percentage of inhibition may be related to the rapid growth of Trichoderma spp., which often completely overlap the colonies of C. truncatum (S2 Fig), L. theobromae (S3 Fig), and M. phaseolina COUFPI 10 (S4 Fig) and COUFPI 11 (S5 Fig). The inhibition may also be related to the efficacy of Trichoderma spp. in competing for space and nutrients and in parasitizing pathogens [45]. S. delphinii COUFPI 209 (S6 Fig) and COUFPI 249 (S7 Sig) were very aggressive when competing with Trichoderma spp. by space and nutrients; in some cases, they even grew on endophyte colonies. Antibiosis is also an action mechanism present in endophytic fungi of the Trichoderma genus, which produce several secondary metabolites with antimicrobial activity used to inhibit the development of plant pathogens [46]. Thus, this action mechanism may also be occurring, justifying the high %inhibition achieved by Trichoderma spp.
Several studies have demonstrated the ability of Trichoderma strains to inhibit the growth of plant pathogens through antibiosis mechanism [46, 47]. Among the secondary metabolites of Trichoderma with antimicrobial activity are syringaresinol [48], HT-2 toxin [49], trigonelline [50], trans-zeatin [51], koninginin A [52], koninginin D [53], koninginin E [52], 6-pentyl-α-pyrone [10], gliotoxin, gliovirin, crisopanol, pyrone, 6-pentyl-2H-pyran-2-one, harzianic acid, koningic acid [53], alamethicin, and dermadin [53].
To investigate the antibiosis mechanism performed by Trichoderma spp. isolates, we also evaluated whether Trichoderma spp. methanolic extracts had inhibitory activity against one of the plant pathogens. For this purpose, we selected the fungus C. truncatum, the causal agent of anthracnose in soybeans, which is economically relevant. As a result, antifungal activity increased with increasing concentrations of the methanolic extracts, potentiating the %inhibition of the pathogen (S8 Fig). At concentrations of 0.5, 1.0, and 2.0 mg mL-1, there was an increase in the %inhibition of C. truncatum when compared to the dose of 0.0 mg mL-1 for all isolates. However, no significant difference from the concentration of 0.5 mg mL-1 was observed for four of the isolates (T. longibrachiatum (UFPIT02), Trichoderma sp. T3 (UFPIT06), Trichoderma sp. T4 (UFPIT08), and T. orientale (UFPIT17)).
The extracts of T. koningiopsis (UFPIT10) and Trichoderma sp. T5 (UFPIT11) showed the highest % inhibition, differing statistically from the other isolates at a concentration of 2 mg mL-1 (S8 Fig). Interestingly, T. koningiopsis (UFPIT10) and Trichoderma sp. T5 (UFPIT11) did not show the highest activities in the pairing co-culture bioassay (Fig 1A), although both presented %inhibition higher than 50%. The divergence of results may be explained by the variation between the performance of Trichoderma isolates of the same species in in vitro and in vivo bioassays, since the biological control mechanisms of fungi can occur simultaneously, affecting their action [21, 22].
Correlation analysis between co-culture and crude extract bioassays indicated that Trichoderma spp. (UFPIT05, UFPIT08, UFPIT11, and UFPIT13), T. koningiopsis (UFPIT07, UFPIT10, UFPIT16, and UFPIT19), T. orientale (UFPIT12 and UFPIT15), and T. longibrachiatum (UFPIT02) had a positive linear relationship, with emphasis on Trichoderma sp. T4 (UFPIT08) and T. longibrachiatum (UFPIT02), which presented r values of 0.97 and 0.96, respectively (S3 Table). On the other hand, Trichoderma sp. T1 (UFPIT04), Trichoderma sp. T3 (UFPIT06), T. koningiopsis (UFPIT03), and T. orientale (UFPIT01, UFPIT09, UFPIT14, UFPIT17, and UFPIT18) showed negative correlations, with emphasis on Trichoderma sp. T3 UFPIT06 (r = -1.00), indicating that other biocontrol mechanisms prevailed in relation to antibiosis.
Untargeted metabolomic analysis revealed antimicrobial metabolites of Trichoderma strains from forest species
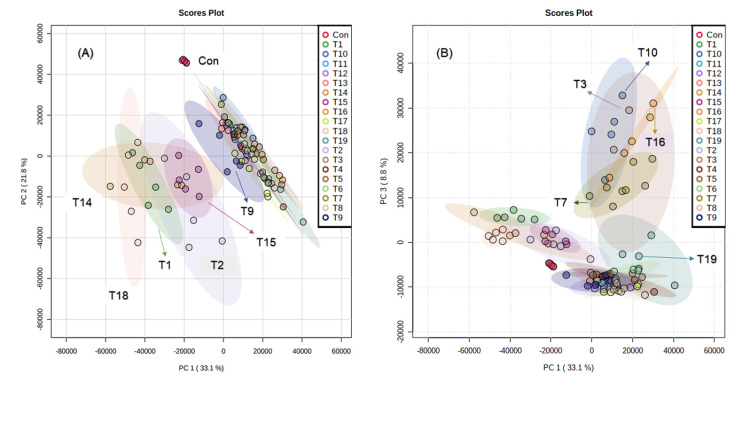
The metabolic content of the methanolic extracts of all Trichoderma spp. isolates (S8 Fig) were explored by PCA to correlate with the efficiency of inhibition. The PCA showed that 54.9% of the total variation in the data were represented by the first two principal components (Fig 2A). Although a great overlap of the species was observed, samples of Trichoderma spp. isolates were clearly separated from the control samples. Clustering of species was performed according to the similarity of their metabolomic profiles and resulted in two large clusters. In the first, with negative scores for PC1, T. longibrachiatum UFPIT02 (T2) and T. orientale UFPIT01 (T1), UFPIT14 (T14), and UFPIT18 (T18) overlapped, and T. orientale UFPIT15 (T15) remained close to this group, overlapping with T. longibrachiatum UFPIT02 (T2) and T. orientale UFPIT14 (T14).
Fig 2. PC1 x PC2 (A) and PC1 x PC3 (B) score plots of metabolic fingerprints of Trichoderma spp. cultures generated using MetaboAnalyst, where Con = Control, UFPIT01 = T1, UFPIT02 = T2, UFPIT03 = T3, UFPIT04 = T4, UFPIT05 = T5, UFPIT06 = T6, UFPIT07 = T7, UFPIT08 = T8, UFPIT09 = T9, UFPIT10 = T10, UFPIT11 = T11, UFPIT12 = T12, UFPIT13 = T13, UFPIT14 = T14, UFPIT15 = T15, UFPIT16 = T16, UFPIT17 = T17, UFPIT18 = T18, and UFPIT19 = T19.
A second clustering, with majority positive scores for PC1, was formed by the species T. orientale (UFPIT12 and UFPIT17) and T. koningiopsi (UFPIT03, UFPIT07, UFPIT10, UFPIT16, and UFPIT19) and unidentified isolates Trichoderma sp. T1 (UFPIT04), Trichoderma sp. T2 (UFPIT05), Trichoderma sp. T3 (UFPIT06), Trichoderma sp. T4 (UFPIT08), Trichoderma sp. T5 (UFPIT11), and Trichoderma sp. T6 (UFPIT13). T. orientale UFPIT09 (T9), located near the zero value of PC1, remained intermediate between these two large clusters of species. The PC1 x PC3 score plot (Fig 2B) revealed some clusters similar to those observed in the PC1 x PC2 score plot; however, a new group stood out, with positive scores for PC1, formed by T. koningiopsis UFPIT03 (T3), UFPIT07 (T7), UFPIT10 (T10), and UFPIT16 (T16), partially overlapping with T. koningiopsis UFPIT19 (T19).
Altogether, PCA showed that Trichoderma spp. from the same species can produce different secondary metabolites, and isolates from different species can produce similar molecules. In the loading plot, the metabolites produced by Trichoderma spp. isolates are displayed, and the most distant points represent the metabolites that most influenced the clustering. Molecular signatures of Trichoderma spp. isolates were identified according to the elution order, MS/MS fragmentation pattern, molecular formula, and database search. A total of 16 molecules were identified (Table 1 and S11 Fig).
Table 1. Secondary metabolites identified in Trichoderma strains using UPLC-ESI-Q-TOF-MS.
| No. | m/z | Retention time (min) | Adduct | MS/MS Fragment masses | Molecular formula | Exact mass | Putative identification | Δ m/z (ppm) | Strains | Biological activity | References |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 264.1086 | 0.68 | [M+H-H2O]+ | 69.0328, 84.0443, 139.0881, 150.9268 | C11H15N5O4 | 281.1119 | 2-O-Methyladenosine | 1.78 | All | Anti-inflammatory | [54] |
| 2 | 118.0863 | 0.86 | [M+H]+ | 59.0705, 60.0838, 99.0061 | C5H11NO2 | 117.0790 | Glycine-Betaine | 0 | All | Plant growth promoter | [55] |
| 3 | 138.0553 | 0.91 | [M+H]+ | 65.0380, 78.0338, 92.0496, 93.0572, 138.0562 | C7H7NO2 | 137.0480 | Trigonelline | -2,19 | UFPIT02-UFPIT05, UFPIT07-UFPIT11, and UFPIT13-UFPIT19 | Plant growth promoter, antibacterial | [56, 57] |
| 4 | 220.1196 | 4.63 | [M+H]+ | 97.0356 | C10H13N5O | 219.1123 | Trans-Zeatin | -1.37 | UFPIT06, UFPIT07, UFPIT10, and UFPIT12 | Plant growth promoter, antibacterial, antifungal | [51, 58–60] |
| 5 | 237.1126 | 6.80 | [M+H-H2O]+ | 215.0709, 235.1137, 249.0980 | C13H18O5 | 254.1159 | Phomalone | -1.96 | All | Antibacterial, antifungal, cytotoxic | [61] |
| 6 | 247.0957 | 10.34 | [M+H]+ | 56.9367, 162.0252, 166.0648 | C14H14O4 | 246.0895 | Columbianetin | -1.22 | UFPIT01, UFPIT02, UFPIT09, UFPIT14, UFPIT15, and UFPIT18 | Antibacterial, antifungal | [62–65] |
| 7 | 265.1423 | 10.55 | [M+H]+ | 173.0774, 189.0489, 195.0886, 245.0917 | C15H20O4 | 264.1352 | Abscisic acid | 3,78 | UFPIT04, and UFPIT13 | Plant growth promoter, antioxidant, antibacterial, antifungal | [66–68] |
| 8 | 419.1713 | 10.83 | [M+H]+ | 186.0949, 204.1036, 441.1527 | C22H26O8 | 418.1640 | Syringaresinol | -2.87 | UFPIT01, UFPIT02, UFPIT13, UFPIT14, UFPIT15, and UFPIT18 | Antibacterial, antifungal, anti-inflammatory | [48, 69] |
| 9 | 281.1754 | 12.28 | [M+H]+ | 123.0814, 133.0655, 160.0524, 175.0431, 177.0254, 245.1556, 263.1660 | C16H24O4 | 280.1688 | Brefeldin-A | -4.64 | UFPIT03, UFPIT07, UFPIT10, UFPIT16, and UFPIT19 | Antiviral, antifungal, antitumoral | [70, 71] |
| 10 | 305.1721 | 13.93 | [M+Na]+ | 147.0131, 153.0918, 161.0294, 225.0098, 255.1513, 259.1615, 276.1381 | C16H26O4 | 282.1837 | Koninginin E | -2.13 | UFPIT03, UFPIT07, UFPIT10, UFPIT16, and UFPIT19 | Plant growth promoter, antifungal | [52] |
| 11 | 307.1882 | 14.11 | [M+Na]+ | 133.0656, 267.1297, 289.1688 | C16H28O4 | 284.1993 | Koninginin A | -1.76 | UFPIT03, UFPIT07, UFPIT10, UFPIT16, and UFPIT19 | Plant growth promoter, Antifungal | [52, 72] |
| 12 | 453.1914 | 14.23 | [M+H-H2O]+ | 147.0929, 154.0670, 174.1137, 212.2395, 225.0931, 263.1654, 281.1769, 328.2860, 373.2012, 374.2914, 413.1963, 431.2048 | C26H30O8 | 470.1947 | Physodic acid | -1.28 | All | Antibacterial | [73] |
| 13 | 447.2001 | 14.43 | [M+Na]+ | 215.0365, 233.0460, 263.0544, 285.1583, 303.1583, 429.1891, 447.1993 | C22H32O8 | 424.2110 | HT-2 Toxin | -3.07 | UFPIT07, and UFPIT10 | Mycotoxin | [74] |
| 14 | 318.3013 | 16.01 | [M+H]+ | 97.9455, 150.0253, 264.2691, 282.2798, 286.2755, 294.2803 | C18H39NO3 | 317.2940 | Phytosphingosine | -3.15 | UFPIT01, UFPIT02, UFPIT09, and UFPIT17 | Anti-inflammatory, antibacterial | [75–77] |
| 15 | 163.0393 | 16.54 | [M+H]+ | 120.9752, 121.0289, 135.0442, 163.0393 | C9H6O3 | 162.0321 | 4-Hydroxycoumarin | -2.47 | All | Antifungal, antibacterial, antioxidant, antitumoral | [78, 79] |
| 16 | 338.3413 | 20.72 | [M+H]+ | 97.1011, 100.0764, 109.1018, 111.0814, 114.0919, 121.1023, 123.0819, 125.0974, 128.1070, 135.1178, 139.0942 | C8H4O3 | 337.3341 | Erucamide | 1.19 | All | Antibacterial | [80] |
The metabolite of m/z 264.10862 was found in all Trichoderma spp. isolates and identified as 2-O-methyladenosine (Table 1, metabolite 1), a member of the adenosine class that has been isolated from the mycelium of Cordyceps sinensis [54]. The metabolite of m/z 118.0863 [M+H]+ was detected in all Trichoderma spp. isolates and identified as glycine-betaine (Table 1, metabolite 2), presenting in the MS/MS spectrum the fragment of m/z 59.0705 corresponding to (CH3)3N+ [55]. Betaines are naturally occurring metabolites fundamental to the mitigation of osmotic stress in plants and macro- and microorganisms [55]. The metabolite of m/z 138.0553 [M+H]+ was identified as trigonelline (Table 1, metabolite 3) and was detected in T. longibrachiatum (UFPIT02), Trichoderma sp. T2 (UFPIT05), T. koningiopsis (UFPIT07), Trichoderma sp. T5 (UFPIT11), Trichoderma sp. T6 (UFPIT13), and T. koningiopsis (UFPIT19) isolates. The MS/MS spectrum was characterized by the fragment of m/z 92.0496 [__HCOOH]+, referring to the carboxylic acid group [56, 57]. This alkaloid is widely used in medicine to protect the liver and heart and to treat hypercholesterolemia [81]. and has already been identified in T. asperellum fermentation cultures [50].
The metabolite of m/z 220.1196 was identified as trans-zeatin (Table 1, metabolite 4), and it was detected in Trichoderma sp. T3 (UFPIT06), T. koningiopsis (UFPIT07 and UFPIT10), and T. orientale (UFPIT12) isolates. This cytokinin, previously reported in Trichoderma strains, can be used for plant growth stimulation and affects host plant phytohormones to enhance plant resistance against pathogens [50, 58]. The metabolite of m/z 237.1126 [M+H]+, identified as phomalone (Table 1, metabolite 5) and detected in all Trichoderma spp., is a common metabolite in many fungal species, with anti-inflammatory, antibacterial, antifungal, and antialgal activities [61]. The metabolite of m/z 247.0957 detected in T. orientale (UFPIT01), T. longibrachiatum (UFPIT02), and T. orientale (UFPIT09, UFPIT14, UFPIT15, and UFPIT18) isolates was identified as columbianetin (Table 1, metabolite 6), a phytoalexin with diverse biological activities [61]. and that has been extracted from cultures of an endophytic strain of Annulohypoxylon ilanense [48].
The metabolite of m/z 265.1423 was identified as abscisic acid (Table 1, metabolite 7), a phytohormone directly involved in plant-microorganism interactions, improving the defense system and plant development [66], and it was detected only in Trichoderma sp. T1 (UFPIT04) and Trichoderma sp. T6 (UFPIT13) isolates. The metabolite of m/z 419.1713 detected in T. orientale (UFPIT01), T. longibrachiatum (UFPIT02), Trichoderma sp. T6 (UFPIT13), and T. orientale (UFPIT14, UFPIT15, and UFPIT18) isolates were identified as syringaresinol (Table 1, metabolite 8), a lignan that has been found to be a secondary metabolite of an endophytic strain of A. ilanense [48]. The metabolite of m/z 281.1754, present in T. koningiopsis (UFPIT03, UFPIT07, UFPIT10, UFPIT16, and UFPIT19), was identified as brefeldin-A (BFA) (Table 1, metabolite 9) and showed MS/MS spectrum with fragments of m/z 263.1660 [M + H-H2O]+ and 245.1556 [M + H - 2H2O]+ formed by the BFA dehydration pathway [70]. This metabolite is an antibiotic already isolated in several fungal genera, such as Alternaria, Ascochyta, Penicillium, Curvularia, Cercospora, and Phyllosticta. BFA has been reported to have important bioactivities, such as antibiotics, antivirals, cytostatics, antimitotics and antitumors [70].
The metabolites of m/z 305.1721 and m/z 307.1882 were identified as koninginin E and koninginin A, respectively (Table 1, metabolites 10 and 11). Koninginins are secondary metabolites belonging to the group of polyketides that are bioactive against several plant pathogens. Koninginin E has already exhibited activity against Gaeumannomyces graminis var. tritici, while koninginin A already exhibited activity against G. graminis var. tritici, F. oxysporum, F. solani and Alternaria panax [52]. Koninginins A and E were detected in T. koningiopsis (UFPIT03, UFPIT07, UFPIT10, UFPIT16, and UFPIT19 isolates). The metabolite of m/z 453.1914 was identified as physodic acid (Table 1, metabolite 12) and was detected in all Trichoderma spp. Physodic acid is a metabolite belonging to the depsidone group, and its antibacterial activity against S. aureus has been previously reported [73]. The metabolite of m/z 447.2001, detected in T. koningiopsis (UFPIT07 and UFPIT10), was identified as HT-2 toxin (Table 1, metabolite 13). The MS/MS spectrum was characterized by fragments of m/z 215.0365 [HT2—isoval acid—acetic acid—H2O - CH2O + H]+, 233.0460 [C14H16O3 + H]+ and 263.0544 [HT2—isoval acid—acetic acid + H]+ [74]. HT-2 toxin is a secondary metabolite found mainly in fungi of the Fusarium genus and is classified as a trichothecene type A mycotoxin [74].
The metabolite of m/z 318.3013 identified as phytosphingosine (Table 1, metabolite 14) presented MS/MS spectrum with fragments of m/z 264.2691 [M+H-DHO-2H2O]+ and 282.2798 [M+H-DHO-H2O]+, formed by the phytosphingosine dehydration pathway [75]. Phytosphingosine is a long-chain sphingolipid present in microorganisms, plants, and some mammalian tissues with antimicrobial and anti-inflammatory activity [76] and was produced by T. orientale (UFPIT01, UFPIT09, and UFPIT17) and T. longibrachiatum (UFPIT02) isolates. The metabolite of m/z 163.0393 detected in all Trichoderma spp. was identified as 4-hydroxycoumarin (Table 1, metabolite 15), which is a fungal metabolite obtained from the precursor coumarin [79] that has important biological activities [78]. The metabolite of m/z 338.3413 detected in all Trichoderma spp. was identified as erucamide (Table 1, metabolite 16) and has been reported in T. longibrachiatum [80].
Altogether, the plethora of and the variety of secondary metabolites identified in the present study highlight how Trichoderma strains are capable of producing metabolites with different biological activities, which makes them very promising not only for the biocontrol of plant diseases but also for their application in medical, pharmaceutical and industrial biotechnology. Forest species from the Cerrado-Caatinga ecotone are rich in genetic resources and have diverse fauna and flora, with enormous biotechnological potential, including their diversity of endophytic fungi [26].
Conclusions
Trichoderma strains from the Cerrado-Caatinga ecotone revealed significant biocontrol potential against crop pathogenic fungi through antibiosis and multiple mechanisms, with possibilities of being used in formulations of biological products for the treatment of plant diseases. Metabolomic analysis proved to be effective in differentiating Trichoderma strains, in addition to identifying a variety of secondary metabolites with antimicrobial activity and other different bioactivities, demonstrating the importance of studying the biological resources of this area, which are still underexplored. Additionally, new bioactive metabolites can still be discovered, since this mutualistic association of endophytic fungi with their hosts is controlled by the genes of both organisms and modulated by the environment in which they live.
Supporting information
Mycelial growth rate index (MGRI) of C. truncatum (A), L. theobromae (B), M. phaseolina COUFPI 10 and COUFPI 11 (C), and S. delphinii COUFPI 209 and COUFPI 249 (D) paired with Trichoderma strains. Means followed by the same letter do not differ from each other by the Scott–Knott test at the 5% probability level. The coefficients of variation (CVs) were 20.51% for L. theobromae, 15.99% for M. phaseolina COUFPI 10, 19.13% for M. phaseolina COUFPI 11, 10.68% for S. delphinii COUFPI 209, and 9.54% for S. delphinii COUFPI 249. Different lowercase letters indicate a significant difference between Trichoderma spp. Different capital letters indicate a significant difference between Trichoderma spp.
(TIF)
(TIF)
(TIF)
(TIF)
(TIF)
(TIF)
(TIF)
The coefficients of variation (CVs) were 4.65% for the concentration of 0.5 mg mL-1, 8.45% for 1.0 mg mL-1 and 9.36% for 2.0 mg mL-1.
(TIF)
PC1 x PC2 (A) and PC1 x PC3 (B) loading plots of metabolic fingerprints of Trichoderma spp. cultures generated using MetaboAnalyst. Con = Control, UFPIT01 = T1, UFPIT02 = T2, UFPIT03 = T3, UFPIT04 = T4, UFPIT05 = T5, UFPIT06 = T6, UFPIT07 = T7, UFPIT08 = T8, UFPIT09 = T9, UFPIT10 = T10, UFPIT11 = T11, UFPIT12 = T12, UFPIT13 = T13, UFPIT14 = T14, UFPIT15 = T15, UFPIT16 = T16, UFPIT17 = T17, UFPIT18 = T18, and UFPIT19 = T19.
(TIF)
(TIF)
(TIF)
(DOCX)
(DOCX)
(DOCX)
Data Availability
All relevant data are within the manuscript and its Supporting Information files.
Funding Statement
F.W.A.S. is supported by the National Council for Scientific and Technological Development (CNPq/PIBIC). C.P.C. and M.N.E. are supported by the MackPesquisa. A.A.R.S is supported by the Coordination for the Improvement of Higher Education Personnel (CAPES, Finance Code 001). A.M.P. is supported by São Paulo Research Foundation (FAPESP, grant number 2019/04314-6). Funding for open access charge: MackPesquisa.
References
- 1.Ghorbanpour M, Omidvari M, Abbaszadeh-Dahaji P, Omidvar R, Kariman K. Mechanisms underlying the protective effects of beneficial fungi against plant diseases. Biol Control. 2018; 117:147–157. doi: 10.1016/j.biocontrol.2017.11.006 [DOI] [Google Scholar]
- 2.Villa F, Cappitelli F, Cortesi P, Kunova A. Fungal biofilms: Targets for the development of novel strategies in plant disease management. Front Microbiol. 2017; 8:654. doi: 10.3389/fmicb.2017.00654 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Nagy K, Zheng C, Bolognesi C, Ádám B. Interlaboratory evaluation of the genotoxic properties of pencycuron, a commonly used phenylurea fungicide. Sci Total Environ. 2019; 647:1052–1057. doi: 10.1016/j.scitotenv.2018.08.067 [DOI] [PubMed] [Google Scholar]
- 4.Das S, Pattanayak S. Integrated disease management on grapes–a pioneer of a reformed movement toward sustainability. Int J Curr Microbiol Appl Sci. 2020; 9:993–1005. doi: 10.20546/ijcmas.2020.905.109 [DOI] [Google Scholar]
- 5.Salim HA, Simon S, Lal AA, Abdulrahman AL. Effectiveness of some integrated disease management factors (IDM) on Fusarium wilt infected tomato. J Sci Agri. 2017; 1:244–248. doi: 10.25081/jsa.2017.v1.820 [DOI] [Google Scholar]
- 6.Legrand F, Picot A, Cobo-Díaz JF, Chen W, Le Floch G. Challenges facing the biological control strategies for the management of Fusarium Head Blight of cereals caused by F. graminearum. Biol Control. 2017; 113:26–38. doi: 10.1016/j.biocontrol.2017.06.011 [DOI] [Google Scholar]
- 7.Leiter É, Gáll T, Csernoch L, Pócsi I. Biofungicide utilizations of antifungal proteins of filamentous ascomycetes: current and foreseeable future developments. Biocontrol. 2017; 62:125–138. doi: 10.1007/s10526-016-9781-9 [DOI] [Google Scholar]
- 8.Kyekyeku JO, Kusari S, Adosraku RK, Bullach A, Golz C, Strohmann C, et al. Antibacterial secondary metabolites from an endophytic fungus, Fusarium solani JK10. Fitoterapia. 2017; 119:108–114. doi: 10.1016/j.fitote.2017.04.007 [DOI] [PubMed] [Google Scholar]
- 9.Yao YQ, Lan F, Qiao YM, Wei JG, Huang RS, Li LB. Endophytic fungi harbored in the root of Sophora tonkinensis Gapnep: diversity and biocontrol potential against phytopathogens. Microbiologyopen. 2017; 6:437. doi: 10.1002/mbo3.437 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Huang X, He J, Yan X, Hong Q, Chen K, He Q, et al. Microbial catabolism of chemical herbicides: microbial resources, metabolic pathways and catabolic genes. Pestic Biochem Physiol. 2017; 143:272–297. doi: 10.1016/j.pestbp.2016.11.010 [DOI] [PubMed] [Google Scholar]
- 11.Macías-Rubalcava ML, Sánchez-Fernández RE. Secondary metabolites of endophytic Xylaria species with potential applications in medicine and agriculture. World J Microbiol Biotechnol. 2017; 33:15. doi: 10.1007/s11274-016-2174-5 [DOI] [PubMed] [Google Scholar]
- 12.Harrison JG & Griffin EA. The diversity and distribution of endophytes across biomes, plant phylogeny and host tissues: how far have we come and where do we go from here? Environmental microbiology. 2020; 22:2107–2123. doi: 10.1111/1462-2920.14968 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Noriler SA, Savi DC, Aluizio R, Palácio-Cortes AM, Possiede YM, Glienke C. Bioprospecting and structure of fungal endophyte communities found in the Brazilian biomes, Pantanal, and Cerrado. Frontiers in microbiology. 2018; 9: 1526. doi: 10.3389/fmicb.2018.01526 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zonas de transição [cited 19 December 2021]. In: World Wide Fund for Nature (WWF) [Internet]. Available from: https://www.wwf.org.br/natureza_brasileira/questoes_ambientais/biomas/bioma_transicao.
- 15.Kark S. Effects of Ecotones on Biodiversity. In: Reference module in life sciences. Oxford, England, Science Direct. 2017; 1–7. [Google Scholar]
- 16.Myers N, Mittermeier RA, Mittermeier CG, Fonseca GA, Kent J. Biodiversity hotspots for conservation priorities. Nature. 2000; 403:853–858. doi: 10.1038/35002501 [DOI] [PubMed] [Google Scholar]
- 17.Biodiversidade do cerrado [cited 19 December 2021]. In: CBC ICMBio [Internet]. Available from: https://www.icmbio.gov.br/cbc/conservacao-da-biodiversidade/biodiversidade.html.
- 18.Caatinga [cited 19 December 2021]. In: Ministério do Meio Ambiente [Internet]. Available from: https://antigo.mma.gov.br/biomas/caatinga.html.
- 19.Lapola DM, Martinelli LA, Peres CA, Ometto JPHB, Ferreira ME, Nobre CA, et al. Pervasive transition of the Brazilian land-use system. Nat Clim Chang. 2013; 4:27–35. doi: 10.1038/NCLIMATE2056 [DOI] [Google Scholar]
- 20.Berg G. Plant–microbe interactions promoting plant growth and health: perspectives for controlled use of microorganisms in agriculture. Applied microbiology and biotechnology. 2009; 84: 11–18. doi: 10.1007/s00253-009-2092-7 [DOI] [PubMed] [Google Scholar]
- 21.Benítez T, Rincón AM, Limón MC, Codon AC. Biocontrol mechanisms of Trichoderma strains. International microbiology. 2004; 7: 249–260. [PubMed] [Google Scholar]
- 22.Druzhinina IS, Seidl-Seiboth V, Herrera-Estrella A, Horwitz BA, Kenerley CM, Monte E. Trichoderma: The genomics of opportunistic success. Nat Rev Microbiol. 2011; 9:749. doi: 10.1038/nrmicro2637 [DOI] [PubMed] [Google Scholar]
- 23.Inglis PW, Mello SC, Martins I, Silva JB, Macêdo K, Sifuentes DN, et al. Trichoderma from Brazilian garlic and onion crop soils and description of two new species: Trichoderma azevedoi and Trichoderma peberdyi. PloS One. 2020; 15: e0228485. doi: 10.1371/journal.pone.0228485 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kredics L, Hatvani L, Naeimi S, Körmöczi P, Manczinger L, Vágvölgyi C, et al. Biodiversity of the genus Hypocrea/Trichoderma in different habitats. In: Biotechnology and biology of Trichoderma. 2014; 3–24. doi: [DOI] [Google Scholar]
- 25.Ma J, Tsegaye E, Li M, Wu B, Jiang X. Biodiversity of Trichoderma from grassland and forest ecosystems in Northern Xinjiang, China. 3 Biotech. 2020; 10: 1–13. doi: 10.1007/s13205-019-1978-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Silva HF, Santos AMG, Santos MVOD, Bezerra JL, Luz EDMN. Seasonal variation in the occurrence of fungi associated with forest species in a Cerrado-Caatinga transition area. Revista Árvore. 2020; 44. doi: 10.1590/1806-908820200000009 [DOI] [Google Scholar]
- 27.Silva HF, Costa EM, Santos AMG, Amaral ACT, Oliveira RJV, Bezerra JL, et al. Molecular identification and phylogenetic analysis of Trichoderma isolates obtained from woody plants of the semi-arid of Northeast Brazil. Nova Hedwigia. 2021; 112:485–500. doi: 10.1127/nova_hedwigia/2021/0622 [DOI] [Google Scholar]
- 28.Silva HF, Santos AMG, Amaral ACT, Bezerra JL, Luz EDMN. Bioprospection of Trichoderma spp. originating from a Cerrado-Caatinga ecotone on Colletotrichum truncatum, in soybean. Revista Brasileira de Ciências Agrárias (Agrária). 2020; 15. doi: 10.5039/agraria.v15i1a7680 [DOI] [Google Scholar]
- 29.Mota JM, Melo MP, Silva FFS, Sousa EMJ, Sousa ES, Barguil BM, et al. Fungal diversity in lima bean seeds. Revista Brasileira de Engenharia de Biossistemas. 2017; 11:79–87. [Google Scholar]
- 30.Severo R, Shibutani LJ, Sousa ES, Matos KS, Beserra Júnior JEA, Melo MP. Sclerotium delphinii causing concentric leaf spots in Piper nigrum in Brazil. Australas Plant Pathol. 2021. doi: 10.1007/s13313-021-00820-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Mariano RLR. Métodos de seleção “in vitro” para controle microbiológico. Revisão Anual de Patologia de Plantas, Passo Fundo. 1993; 1:369–409. [Google Scholar]
- 32.Camporota P. Antagonism in vitro of Trichoderma spp. vis-a-vis Rhizoctonia solani Kuhln. Agronomie. 1985; 5:613–620. [Google Scholar]
- 33.Oliveira JA. Efeito do tratamento fungicida em sementes no controle de tombamento de plântulas de pepino (Cucumis sativa L.) e pimentão (Capsicum annanum L.). M.Sc. Thesis, Federal University of Lavras. 1991. Available from: http://repositorio.ufla.br/handle/1/33483. [Google Scholar]
- 34.Fernandes EG, Pereira OL, Silva CC, Bento CBP, Queiroz MV. Diversity of endophytic fungi in Glycine max. Microbiol Res. 2015; 181:84–92. doi: 10.1016/j.micres.2015.05.010 [DOI] [PubMed] [Google Scholar]
- 35.Tam EW, Chen JH, Lau EC, Ngan AH, Fung KS, Lee KC, et al. Misidentification of Aspergillus nomius and Aspergillus tamarii as Aspergillus flavus: characterization by internal transcribed spacer, β-tubulin, and calmodulin gene sequencing, metabolic fingerprinting, and matrix-assisted laser desorption ionization–time of flight mass spectrometry. J Clin Microbiol. 2014; 52: 1153–1160. doi: 10.1128/JCM.03258-13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Yadav M, Dubey MK, Upadhyay RS. Systemic Resistance in Chilli Pepper against Anthracnose (Caused by Colletotrichum truncatum) induced by Trichoderma harzianum, Trichoderma asperellum and Paenibacillus dendritiformis. Journal of Fungi. 2021; 7: 307. doi: 10.3390/jof7040307 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Begum MM, Sariah M, Abidin ZMA, Puteh AB, Rahman MA. Antagonistic potential of selected fungal and bacterial biocontrol agents against Colletotrichum truncatum of soybean seeds. Pertanica J. Trop. Agric. Sci. 2008; 31: 45–53. [Google Scholar]
- 38.Jagtap GP, Gavate DS, Dey U. Control of Colletotrichum truncatum causing anthracnose/pod blight of soybean by aqueous leaf extracts and biocontrol agents. Legume Research-An International Journal. 2014; 37: 329–334. doi: 10.5958/j.0976-0571.37.3.050 [DOI] [Google Scholar]
- 39.Wanjiku EK, Waceke JW, Mbaka JN. Suppression of Stem-End Rot on Avocado Fruit Using Trichoderma spp. in the Central Highlands of Kenya. Advances in Agriculture. 2021; 2021. d doi: 10.1155/2021/8867858 [DOI] [Google Scholar]
- 40.Bhadra M, Khair A, Hossain MA, Sikder MM. Efficacy of Trichoderma spp. and fungicides against Lasiodiplodia theobromae. Bangladesh Journal of Scientific and Industrial Research. 2014; 49: 125–130. doi: 10.3329/bjsir.v49i2.22008 [DOI] [Google Scholar]
- 41.Thangavelu R, Sangeetha G, Mustaffa MM. Cross-infection potential of crown rot pathogen (Lasiodiplodia theobromae) isolates and their management using potential native bioagents in banana. Australasian Plant Pathology. 2007; 36: 595–605. [Google Scholar]
- 42.Sridharan AP, Sugitha T, Karthikeyan G, Nakkeeran S, Sivakumar U. Metabolites of Trichoderma longibrachiatum EF5 inhibits soil borne pathogen, Macrophomina phaseolina by triggering amino sugar metabolism. Microbial Pathogenesis. 2021; 150: 104714. doi: 10.1016/j.micpath.2020.104714 [DOI] [PubMed] [Google Scholar]
- 43.Saravanakumar K & Wang M. Isolation and molecular identification of Trichoderma species from wetland soil and their antagonistic activity against phytopathogens. Physiological and Molecular Plant Pathology. 2020; 109: 101458. doi: 10.1016/j.pmpp.2020.101458 [DOI] [Google Scholar]
- 44.Swain H, Adak T, Mukherjee AK, Sarangi S, Samal P, Khandual A, et al. Seed biopriming with Trichoderma strains isolated from tree bark improves plant growth, antioxidative defense system in rice and enhance straw degradation capacity. Frontiers in Microbiology. 2021; 12: 240. doi: 10.3389/fmicb.2021.633881 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kottb M, Gigolashvili T, GRoßkinsky DK, Piechulla B. Trichoderma volatiles effecting Arabidopsis: from inhibition to protection against phytopathogenic fungi. Front Microbiol. 2015; 6:995. doi: 10.3389/fmicb.2015.00995 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Mendoza JLH, Pérez MIS, Prieto JMG, Velásquez JDQ, Olivares JGG, Langarica HRG. Antibiosis of Trichoderma spp. strains native to northeastern Mexico against the pathogenic fungus Macrophomina phaseolina. Braz J Microbiol. 2015; 46:1093–1101. doi: 10.1590/S1517-838246420120177 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Khaledi N, Taheri P. Biocontrol mechanisms of Trichoderma harzianum against soybean charcoal rot caused by Macrophomina phaseolina. J Plant Prot Res. 2016; 56:21–31. doi: 10.1515/jppr-2016-0004 [DOI] [Google Scholar]
- 48.Cheng M.J.; Wu M.D.; Chen J.J.; Hsieh S.Y.; Yuan G.F.; Chen I.S.; et al. Secondary metabolites from the endophytic fungus of Annulohypoxylon ilanense. Chem. Nat. Compd. 2013, 49, 523–525. doi: 10.1007/s10600-013-0658-1 [DOI] [Google Scholar]
- 49.Tian Y, Yu D, Liu N, Tang Y, Yan Z, Wu A. Confrontation assays and mycotoxin treatment reveal antagonistic activities of Trichoderma and the fate of Fusarium mycotoxins in microbial interaction. Environmental Pollution. 2020; 267: 115559. doi: 10.1016/j.envpol.2020.115559 [DOI] [PubMed] [Google Scholar]
- 50.Vinci G, Cozzolino V, Mazzei P, Monda H, Spaccini R, Piccolo A. An alternative to mineral phosphorus fertilizers: The combined effects of Trichoderma harzianum and compost on Zea mays, as revealed by 1H NMR and GC–MS metabolomics. PloS One. 2018; 13: e0209664. doi: 10.1371/journal.pone.0209664 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Ni M, Wu Q, Wang GS, Liu QQ, Yu MX, Tang J. Analysis of metabolic changes in Trichoderma asperellum TJ01 at different fermentation time-points by LC-QQQ-MS. J Environ Sci Health, Part B. 2019; 54:20–26. doi: 10.1080/03601234.2018.1507227 [DOI] [PubMed] [Google Scholar]
- 52.Khan RAA, Najeeb S, Hussain S, Xie B, Li Y. Bioactive secondary metabolites from Trichoderma spp. against phytopathogenic fungi. Microorganisms. 2020; 8:817. doi: 10.3390/microorganisms8060817 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Reino JL, Guerrero RF, Hernández-Galán R, Collado IG. Secondary metabolites from species of the biocontrol agent Trichoderma. Phytochem Rev. 2008; 7:89–123. [Google Scholar]
- 54.Yang ML, Kuo PC, Hwang TL, Wu TS. Anti-inflammatory principles from Cordyceps sinensis. Journal of Natural Products. 2011; 74:1996–2000. doi: 10.1021/np100902f [DOI] [PubMed] [Google Scholar]
- 55.Wood KV, Bonham CC, Miles D, Rothwell AP, Peel G, Wood BC, et al. Characterization of betaines using electrospray MS/MS. Phytochemistry. 2002; 59:759–765. doi: 10.1016/s0031-9422(02)00049-3 [DOI] [PubMed] [Google Scholar]
- 56.Liu MH, Tong X, Wang JX, Zou W, Cao H, Su WW. Rapid separation and identification of multiple constituents in traditional Chinese medicine formula Shenqi Fuzheng Injection by ultra-fast liquid chromatography combined with quadrupole-time-of-flight mass spectrometry. J Pharm Biomed Anal. 2013; 74:141–155. doi: 10.1016/j.jpba.2012.10.024 [DOI] [PubMed] [Google Scholar]
- 57.Lu X, Zheng Y, Wen F, Huang W, Chen X, Ruan S, et al. Study of the active ingredients and mechanism of Sparganii rhizoma in gastric cancer based on HPLC-Q-TOF–MS/MS and network pharmacology. Sci Rep. 2021; 11:1–17. doi: 10.1038/s41598-020-79139-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Illescas M, Pedrero-Méndez A, Pitorini-Bovolini M, Hermosa R, Monte E. Phytohormone Production Profiles in Trichoderma Species and Their Relationship to Wheat Plant Responses to Water Stress. Pathogens. 2021; 10:991, 2021. doi: 10.3390/pathogens10080991 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Großkinsky D, Edelsbrunner K, Pfeifhofer H, Van der Graaff E, Roitsch T. Cis- and trans-zeatin differentially modulate plant immunity. Plant Signaling & Behavior. 2013; 8:e24798. doi: 10.4161/psb.24798 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Ahmed EA, Hassan EA, El Tobgy KMK, Ramadan EM. Evaluation of rhizobacteria of some medicinal plants for plant growth promotion and biological control. Annals of Agricultural Sciences. 2014; 59:273–280. doi: 10.1016/j.aoas.2014.11.016 [DOI] [Google Scholar]
- 61.Zhong TH, Zeng XM, Feng SB, Zhang HT, Zhang YH, Luo ZH, et al. Three new phomalone derivatives from a deep-sea-derived fungus Alternaria sp. MCCC 3A00467. Nat Prod Res. 2020; 1–5. doi: 10.1080/14786419.2020.1771706 [DOI] [PubMed] [Google Scholar]
- 62.Zhang L, Ge Y, Li J, Hao J, Wang H, He J, et al. Simultaneous determination of columbianetin-β-d-glucopyranoside and columbianetin in a biological sample by high-performance liquid chromatography with fluorescence detection and identification of other columbianetin-β-d-glucopyranoside metabolites by ultra high-performance liquid chromatography coupled with quadrupole-time of flight mass spectrometry. J Pharm Biomed Anal. 2018; 153:221–231. doi: 10.1016/j.jpba.2018.02.055 [DOI] [PubMed] [Google Scholar]
- 63.Nicoletti R, Fiorentino A. Plant bioactive metabolites and drugs produced by endophytic fungi of Spermatophyta. Agriculture. 2015; 5:918–970. [Google Scholar]
- 64.Zhu JJ & Jiang JG. Pharmacological and nutritional effects of natural coumarins and their structure–activity relationships. Molecular nutrition & food research. 2018; 62:1701073. doi: 10.1002/mnfr.201701073 [DOI] [PubMed] [Google Scholar]
- 65.Souza SM, Delle Monache F, Smânia A. Antibacterial activity of coumarins. Zeitschrift fuer Naturforschung C. 2005; 60:693–700. doi: 10.1515/znc-2005-9-1006 [DOI] [PubMed] [Google Scholar]
- 66.Guzmán-Guzmán P, Porras-Troncoso MD, Olmedo-Monfil V, Herrera-Estrella A. Trichoderma species: versatile plant symbionts. J Phytopathol. 2019; 109:6–16. doi: 10.1094/PHYTO-07-18-0218-RVW [DOI] [PubMed] [Google Scholar]
- 67.Thiruvengadam M, Baskar V, Kim SH, Chung IM. Effects of abscisic acid, jasmonic acid and salicylic acid on the content of phytochemicals and their gene expression profiles and biological activity in turnip (Brassica rapa ssp. rapa). Plant Growth Regulation. 2016; 80:377–390. doi: 10.1007/s10725-016-0178-7 [DOI] [Google Scholar]
- 68.Phukhamsakda C, Macabeo APG, Yuyama KT, Hyde KD, Stadler M. Biofilm inhibitory abscisic acid derivatives from the plant-associated Dothideomycete fungus, Roussoella sp. Molecules. 2018; 23:2190. doi: 10.3390/molecules23092190 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Oh JH, Joo YH, Karadeniz F, Ko J, Kong CS. Syringaresinol inhibits UVA-induced MMP-1 expression by suppression of MAPK/AP-1 signaling in HaCaT keratinocytes and human dermal fibroblasts. International journal of molecular sciences. 2020; 21:3981. doi: 10.3390/ijms21113981 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Fang M, Wang J, Huang Y, Zhao Y. Rapid Screening and Identification of Brefeldin A in Endophytic Fungi Using HPLC–MS/MS. Frontiers of Chemistry in China. 2006; 1:15–19. [Google Scholar]
- 71.Anadu NO, Davisson VJ, Cushman M. Synthesis and anticancer activity of brefeldin A ester derivatives. Journal of medicinal chemistry. 2006; 49: 3897–3905. doi: 10.1021/jm0602817 [DOI] [PubMed] [Google Scholar]
- 72.Li T, Tang J, Karuppiah V, Li Y, Xu N, Chen J. Co-culture of Trichoderma atroviride SG3403 and Bacillus subtilis 22 improves the production of antifungal secondary metabolites. Biological Control. 2020; 140: 104122. doi: 10.1016/j.biocontrol.2019.104122 [DOI] [Google Scholar]
- 73.Dieu A, Mambu L, Champavier Y, Chaleix V, Sol V, Gloaguen V, et al. Antibacterial activity of the lichens Usnea florida and Flavoparmelia caperata (Parmeliaceae). Nat Prod Res. 2020; 34:3358–3362. doi: 10.1080/14786419.2018.1561678 [DOI] [PubMed] [Google Scholar]
- 74.Meng-Reiterer J, Varga E, Nathanail AV, Bueschl C, Rechthaler J, McCormick SP, et al. Tracing the metabolism of HT-2 toxin and T-2 toxin in barley by isotope-assisted untargeted screening and quantitative LC-HRMS analysis. Anal Bioanal Chem. 2015; 407:8019–8033. doi: 10.1007/s00216-015-8975-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Dapic I, Brkljacic L, Jakasa I, Kobetic R. Characterization of ceramides with phytosphingosine backbone by hydrogen-deuterium exchange mass spectrometry. Croat Chem Acta. 2019; 92:1E–1E. doi: 10.5562/cca3506 [DOI] [Google Scholar]
- 76.Kondo N, Ohno Y, Yamagata M, Obara T, Seki N, Kitamura T, et al. Identification of the phytosphingosine metabolic pathway leading to odd-numbered fatty acids. Nat Commun. 2014; 5:1–13. doi: 10.1038/ncomms6338 [DOI] [PubMed] [Google Scholar]
- 77.Choi HK, Cho YH, Lee EO, Kim JW, Park CS. Phytosphingosine enhances moisture level in human skin barrier through stimulation of the filaggrin biosynthesis and degradation leading to NMF formation. Archives of dermatological research. 2017; 309: 795–803. doi: 10.1007/s00403-017-1782-8 [DOI] [PubMed] [Google Scholar]
- 78.Kaur J, Famta P, Khurana N, Vyas M, Khatik GL. Biomedical applications of 4-hydroxycoumarin as a fungal metabolite and its derivatives. New Fut Devel Microbiol Biotech Bioeng. Elsevier. 2020; 209–218. doi: [DOI] [Google Scholar]
- 79.Obaiah N, Bodke YD, Telkar S. Synthesis of 3‐[(1H‐Benzimidazol‐2‐ylsulfanyl)(aryl) methyl]‐4‐hydroxycoumarin derivatives as potent bioactive molecules. ChemistrySelect. 2020; 5:178–184. doi: 10.1002/slct.201903472 [DOI] [Google Scholar]
- 80.Xie Y, Peng Q, Ji Y, Xie A, Yang L, Mu S, et al. Isolation and identification of antibacterial bioactive compounds from Bacillus megaterium L2. Front Microbiol. 2021; 12. doi: 10.3389/fmicb.2021.645484 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Farid MM, Yang X, Kuboyama T, Tohda C. Trigonelline recovers memory function in Alzheimer’s disease model mice: evidence of brain penetration and target molecule. Sci Rep. 2020; 10:1–10. doi: 10.1038/s41598-019-56847-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Mycelial growth rate index (MGRI) of C. truncatum (A), L. theobromae (B), M. phaseolina COUFPI 10 and COUFPI 11 (C), and S. delphinii COUFPI 209 and COUFPI 249 (D) paired with Trichoderma strains. Means followed by the same letter do not differ from each other by the Scott–Knott test at the 5% probability level. The coefficients of variation (CVs) were 20.51% for L. theobromae, 15.99% for M. phaseolina COUFPI 10, 19.13% for M. phaseolina COUFPI 11, 10.68% for S. delphinii COUFPI 209, and 9.54% for S. delphinii COUFPI 249. Different lowercase letters indicate a significant difference between Trichoderma spp. Different capital letters indicate a significant difference between Trichoderma spp.
(TIF)
(TIF)
(TIF)
(TIF)
(TIF)
(TIF)
(TIF)
The coefficients of variation (CVs) were 4.65% for the concentration of 0.5 mg mL-1, 8.45% for 1.0 mg mL-1 and 9.36% for 2.0 mg mL-1.
(TIF)
PC1 x PC2 (A) and PC1 x PC3 (B) loading plots of metabolic fingerprints of Trichoderma spp. cultures generated using MetaboAnalyst. Con = Control, UFPIT01 = T1, UFPIT02 = T2, UFPIT03 = T3, UFPIT04 = T4, UFPIT05 = T5, UFPIT06 = T6, UFPIT07 = T7, UFPIT08 = T8, UFPIT09 = T9, UFPIT10 = T10, UFPIT11 = T11, UFPIT12 = T12, UFPIT13 = T13, UFPIT14 = T14, UFPIT15 = T15, UFPIT16 = T16, UFPIT17 = T17, UFPIT18 = T18, and UFPIT19 = T19.
(TIF)
(TIF)
(TIF)
(DOCX)
(DOCX)
(DOCX)
Data Availability Statement
All relevant data are within the manuscript and its Supporting Information files.