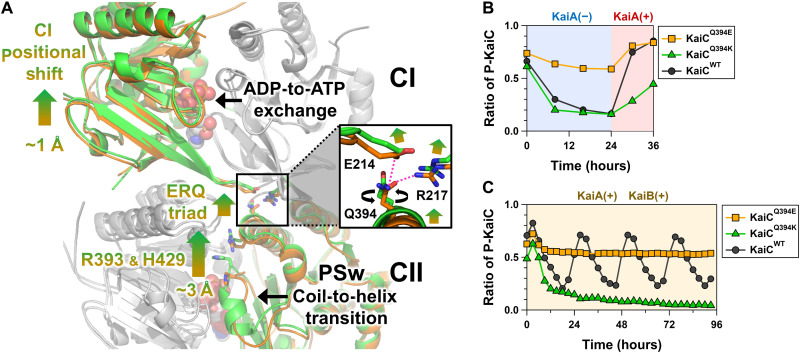
Fig. 3. Structural basis for master allostery between CI-ATPase and CII-PSw in KaiC.
(A) Tertiary and quaternary structural changes in the transition from KaiC-pST (orange) to KaiC-ST (green). The ADP-ATP exchange occurring at the CI-CI interfaces and the coil-to-helix transition for PSw are allosterically coupled via rearrangements of E214, R217, Q394, R393, and H429. The inserted box indicates the zoomed-in view of the ERQ triad mediating the communication between the CI and CII domains. (B) Effects of Q394E and Q394K mutations on the switching ability of CI-CII allostery. Proportion of phosphorylated KaiC (P-KaiC) was measured for 24 hours at 30°C and after KaiA addition at 24 hours. (C) Effects of Q394E and Q394K mutations on the P-cycle.