Table 1.
Optimization of reaction conditions.

| |||||
|---|---|---|---|---|---|
| entry | Az | Base | [M] | 2 | Yield (%)a,b |
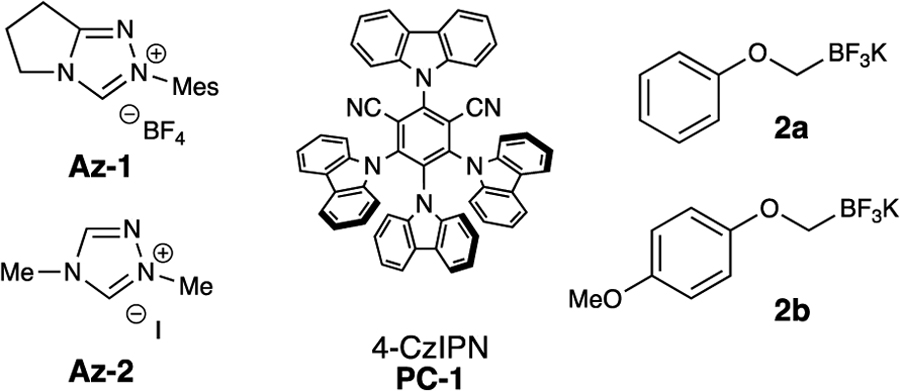
| 1 | 1 | Cs2CO3 | 0.1 | 2a | 29 |
| 2 | 2 | Cs2CO3 | 0.1 | 2a | 20 |
| 3 | 1 | CsOAc | 0.1 | 2a | 32 |
| 4 | 1 | K2CO3 | 0.1 | 2a | 22 |
| 5 | 1 | CsOAc | 0.1 | 2b | 48c |
| 6 | 1 | CsOAc | 0.05 | 2b | 48c |
| 7 | 1 | CsOAc | 0.1 | 2b | 68d |
| 8 | 1 | CsOAc | 0.05 | 2b | 97 (90)d |
| 9 | 1 | CsOAc | 0.05 | 2b | tracee |
| 10 | 1 | CsOAc | 0.05 | 2b | 0f |
| 11 | - | CsOAc | 0.05 | 2b | (30) |
| 12 | 1 | CsOAc | 0.05 | 2b | 0g |
| |||||
Reaction conditions unless otherwise stated: 1a (0.3 mmol), 2 (0.1 mmol), Az (0.015 mmol), base (0.2 mmol), styrene 3a (0.3 mmol), PC (5 μmol), and solvent (0.1 M) for 24 h.
1H NMR yield using 1,3,5-trimethoxybenzene as internal standard (0.33 equiv.) and yield of isolated product is given in parenthesis.
1a (0.3 mmol), 2 (0.2 mmol), 3a (0.1 mmol).
1a (0.3 mmol), 2 (0.5 mmol), 3a (0.1 mmol).
same conditions as [d] except benzoyl fluoride used as acyl starting material (0.3 mmol).
absence of PC-1.
no light.
