FIG 1.
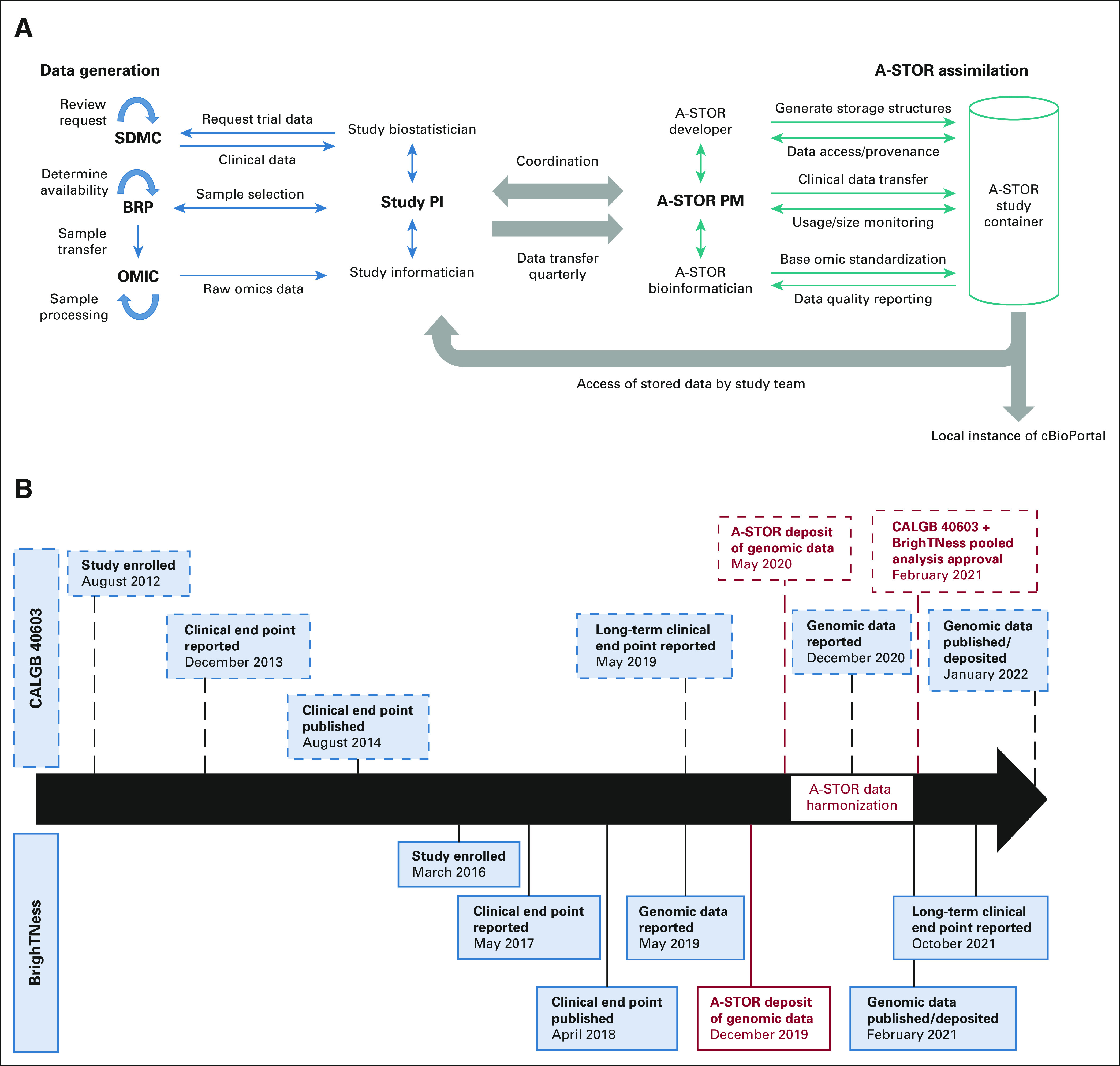
Alliance Standardized Translational Omics Resource (A-STOR) workflow and data timelines. (A) A-STOR workflow and roles. (B) Study and genomic data timelines for CALGB 40603 and BrighTNess, phase III clinical trials of neoadjuvant chemotherapy for triple-negative breast cancer. Dates of key study milestones (enrollment, end point reporting, and results publication) and genomic data milestones (genomic data reporting, publication, and public deposition) are noted. Dates of A-STOR milestones (indicated in red) demonstrate deposition, processing/harmonization, and pooled analysis approval at the accelerated timetable relative to public data deposition. CALGB 40603 milestones are indicated by dashed lines; BrighTNess milestones are indicated by solid lines. A-STOR, Alliance Standardized Translational Omics Resource; BRP, Biorepository; OMIC, Designated Sequencing Core; PI, Principal Investigator; PM, Project Manager; SDMC, Statistical and Data Management Center.
