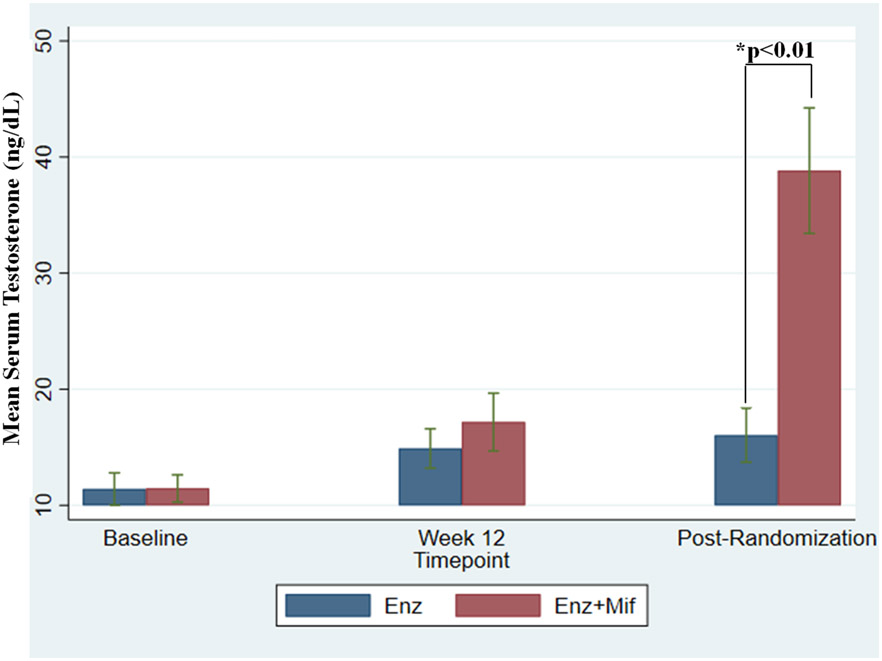
Figure 4: Effect of treatment on serum testosterone levels.
Combination dosing with Enz+Mif did not begin until after week 12 (which represents at least 12 weeks from baseline). In situations in which cycle 1 day 1 data was not available for baseline, the date of the pre-study PSA was used for dating testosterone baseline if patients were screened and started within a 5-day window. Post randomization data represents first testosterone value at least 4 weeks after week 12 testosterone level. Baseline testosterone levels were in the castrate range and did not significantly increase between baseline and Week 12. Combination dosing with Enz+Mif did not start until after week 12. Testosterone increased markedly after the addition of Mif in the Enz+Mif arm due to increased adrenal testosterone production. Testosterone did not substantially change for the group continuing to receive Enz alone. Star (*) represents p<0.01 difference between Enz and Enz+Mif at post-randomization time point.
Abbreviations: Enz, Enzalutamide; Enz alone, Enz 160mg daily after 12-week enzalutamide monotherapy lead in; Enz+Mif, Enz 120mg and Mif 300 mg daily after 12 week Enz lead-in; Mif, Mifepristone