Figure 5. A role for late stomatal closure in bacterial pathogenesis.
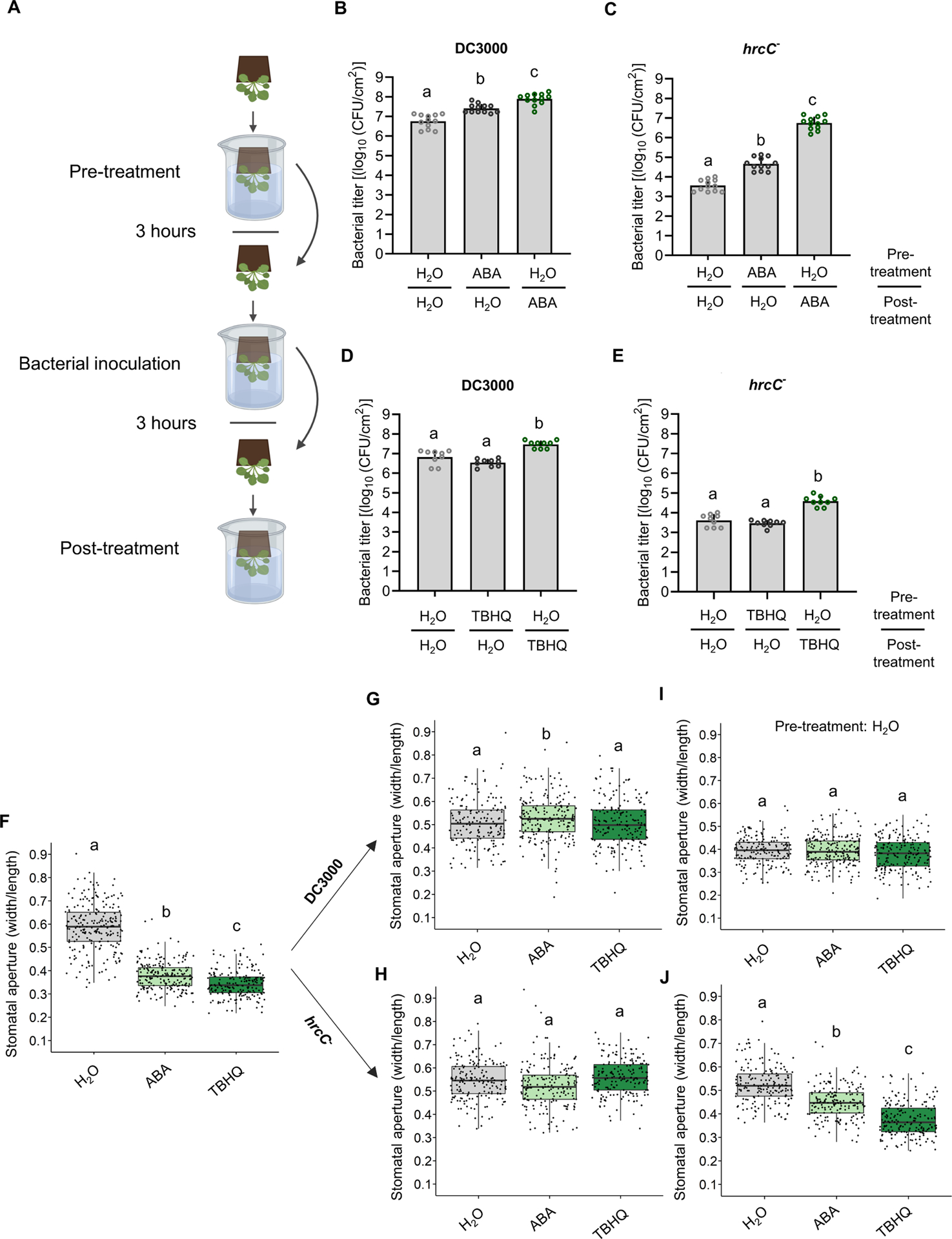
(A) Schematic diagram depicting the experimental design setup to evaluate the importance of pre- vs post-infection stomatal closure. Created with BioRender. (BE) All plants were subjected to three dip treatments. In the Pre-treatment, plants were first dipped in a solution containing either 0.05% ethanol plus 0.025% Silwet L-77 in water (H2O) or the same solution also containing either ABA (30 μM) or TBHQ (100 μM). Three hours after pre-treatment, plants were dip-inoculated with a solution of 2 × 108 CFU/ml of Pst DC3000 or hrcC−, as indicated. All plants were subsequently dip-inoculated in H2O, ABA or TBHQ solutions, as in the pretreatment (Post-treatment). (B-E) Bacterial titers were evaluated 3 days post infection (dpi). Bacterial titers are shown from plants subjected to different combinations of pre- and post-treatments, as indicated, for DC3000 (B and D) or hrcC− (C and E). Data are represented as mean of total experimental replicates, ± SEM. (F-J) Stomatal apertures measured in WT Arabidopsis plants treated as in (B-E). Apertures were measured three hours after pre-treatment with H2O, ABA or TBHQ (F). (G-H) Plants were pre-treated with H2O, ABA or TBHQ, as indicated, followed by bacterial inoculation. Stomatal apertures were measured three hours after inoculation with Pst DC3000 (G) or with hrcC− (H). (I-J) Plants were pretreated with H2O only, followed by inoculation with Pst DC3000 (I) or with hrcC− (J) and Post-treatment with H2O, ABA or TBHQ, as indicated. Stomatal apertures were measured three hours after post-treatment. Data are represented as median values, with minimum and maximum values indicated (F-J). Different letters indicate statistically significant differences, P < 0.05, one-way ANOVA.
