FIGURE 1.
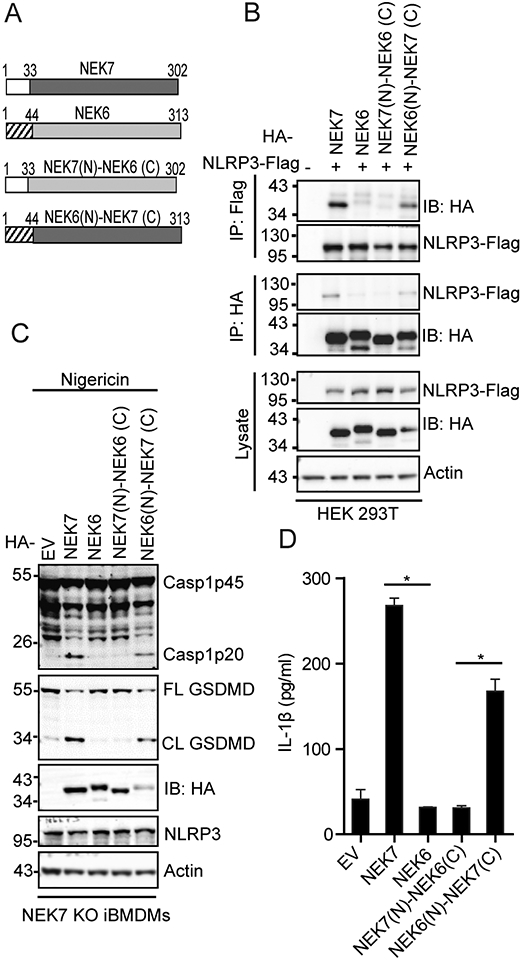
The catalytic domain of NEK7 mediates NLRP3 binding and inflammasome activation. (A) Schematic presentation for domains of NEK7, NEK6, and NEK7-NEK6 chimeras. The N-terminal domain of NEK7 contains the first 33 amino acid residues. The N-terminal domain of NEK6 contains the first 44 amino acid residues. The left amino acid residues comprise the catalytic domains for NEK7 and NEK6, respectively. (B) HA-tagged mouse NEK7, NEK6, or NEK7-NEK6 chimera was co-expressed with Flag-tagged NLRP3 in HEK 293T cells. Cell lysates were immunoprecipitated with anti-Flag or anti-HA antibody and immunoblotted with indicated antibodies. (C) NEK7 KO iBMDMs were reconstituted with HA-tagged NEK7, NEK6, or NEK7-NEK6 chimera by lentiviral transduction. Macrophages were stimulated with 5 μM nigericin for 1 h after LPS priming. Mixtures of cell lysates and supernatants were immunoblotted with indicated antibodies. EV, empty vector. Actin was used as an internal control. (D) Measurement of IL-β in the supernatants from nigericin-stimulated macrophages by ELISA. Data are representative of three experiments. Error bars in D show the SEM of triplicate wells. *p < 0.05.
