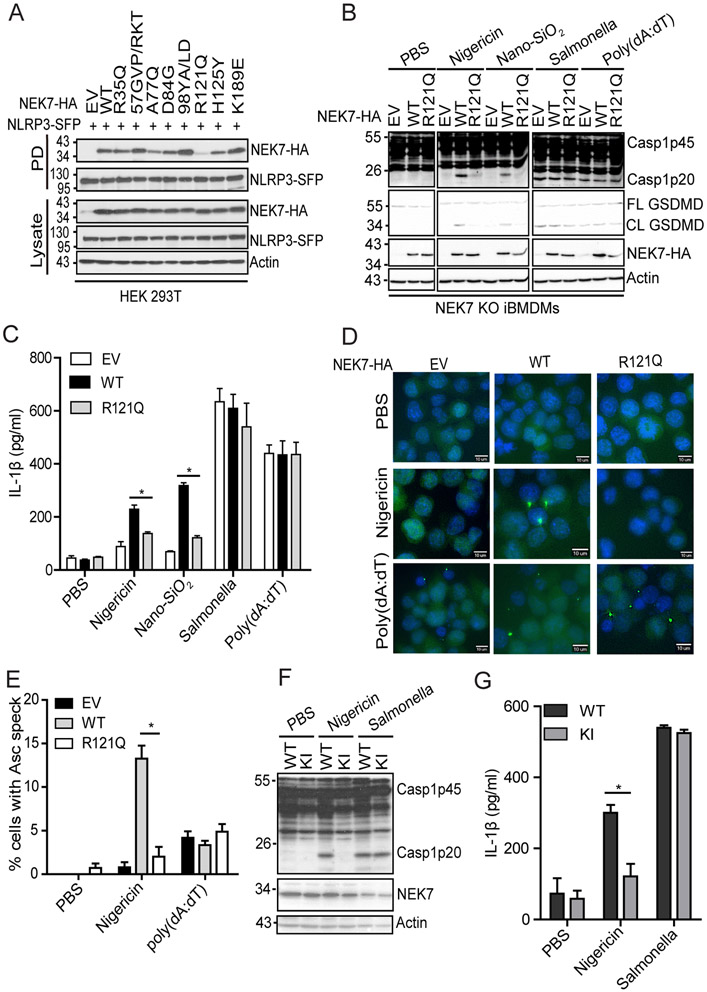
FIGURE 2.
The residue R121 of NEK7 is critical for NLRP3 binding and inflammasome activation. (A) SFP-tagged NLRP3 (NLRP3-SFP) was co-expressed with HA-tagged NEK7 mutants containing swapped residue(s) from NEK6 at the corresponding position(s) in HEK 293T cells. Cell lysates were pulled downed (PD) with streptavidin beads and immunoblotted with indicated antibodies. EV, empty vector. (B) NEK7 KO iBMDMs were reconstituted with wild-type NEK7 or NEK7 mutant (R121Q) by lentiviral transduction. Macrophages were primed with LPS and stimulated with PBS (mock), 5 μM nigericin (1 h), 200 μg/ml Nano-SiO2 (4 h), 4 μg/ml poly(dA:dT) (4 h) or Salmonella (m.o.i= 10, 2h). Mixtures of cell lysates and supernatants were immunoblotted with indicated antibodies. (C) Measurement of IL-β in the supernatants from stimulated macrophages by ELISA. (D) ASC immunostaining in wild-type or mutant NEK7-reconstituted macrophages primed with LPS and stimulated with PBS, nigericin, or poly(dA:dT). Scale bars, 10 μm. (E) Quantification of ASC specks from D. The percentage of ASC speck-containing cells was calculated from three different fields with at least 100 cells each. (F) Parent (WT) or NEK7R121Q knock-in mutant (KI) macrophages were stimulated with PBS, nigericin (5 μM, 1h), or Salmonella (m.o.i=10, 2h) after LPS priming. Mixtures of cell lysates and supernatants were immunoblotted with indicated antibodies. (G) Measurement of IL-β in the supernatants from the stimulated parent (WT) or NEK7R121Q knock-in mutant (KI) macrophages by ELISA. Actin was used as an internal control. Data are representative of three experiments. Error bars in C, E, and G show the SEM of triplicates. *p < 0.05.