Figure 1.
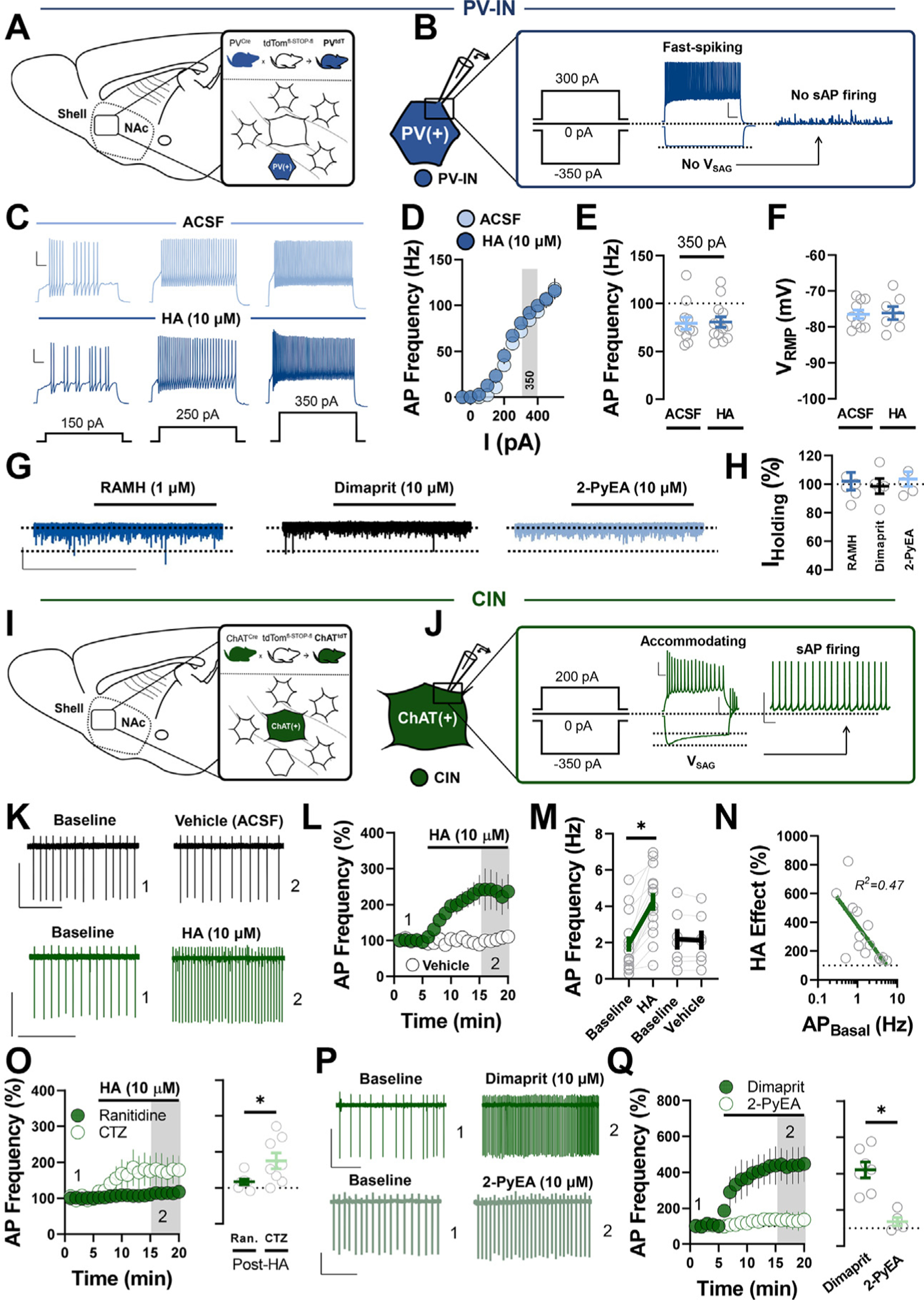
HA differentially regulates PV-IN and CIN excitability in the NAc shell. (A) Schematic depicting transgenic reporter strategy labeling tdT-positive PV-INs in the NAc shell of PVtdT mice. (B) Representative traces of PV-IN AP firing (left), membrane hyperpolarization (middle), and absent sAP firing (right) following +300 pA, −350 pA, and 0 pA current injection, respectively (scale bar = 20 mV/100 ms). (C) Representative traces of PV-IN AP firing evoked via +150 pA, +250 pA, and +350 pA current injection in ACSF (top, light blue) and HA (bottom, dark blue) (scale bar = 20 mV/100 ms). (D) Input-output curve of PV-IN AP firing in ACSF and HA. (E) Quantification of AP frequency in ACSF or HA following 350 pA current injection (AP350-pA ACSF: 80.58 ± 5.43 Hz, n = 11; AP350-pA HA: 79.31 ± 6.36 Hz, n =13, p = .880). (F) VRMP of PV-INs in ACSF vs. HA (VRMP ACSF: −76.50 ± 1.18 mV, n = 10; VRMP HA: −76.11 ± 1.81 mV, n = 7, p = .853). (G) Representative traces of IHolding in PV-INs exposed to RAMH (dark blue), dimaprit (black), or 2-PyEA (light blue) (scale bar = 30 pA/4 min). (H) Postdrug IHolding normalized within-cell to baseline (ACSF) IHolding RAMH: 102.01% ± 6.23%, n = 6; folding dimaprit: 98.60% ± 6.28%, n = 5; IHding 2-PyEA: 103.60% ± 5.08%, n = 5; one-way analysis of variance, F2,13 = 0.19, p = .829). (I) Schematic depicting transgenic reporter strategy labeling tdT-positive CINs in the NAc shell of ChATtdT mice. (J) Representative traces of CIN accommodating APs (scale bar = 20 mV/100 ms), hyperpolarization-activated Vsag (scale bar = 50 mV/100 ms), and tonic sAP firing (scale bar = 50 mV/500 ms), following +200 pA, −350 pA, and 0 pA current injection, respectively. (K) Representative traces of CIN sAP firing in pre- and post-vehicle (ACSF) (black) or HA (green) (scale bars = 50 pA/3 s). (L) Normalized time-course summary of sAP firing frequency at t(gray) following HA or vehicle application. (M) Average sAP frequency before and after HA or ACSF at t(gray) (baseline: 1.93 ± 0.42 Hz, HA: 4.256 ± 0.49 Hz, n = 14, p < .0001; baseline: 2.185 ± 0.58 Hz, vehicle: 2.14 ± 0.53 Hz, n = 7, p = .511). (N) Semilogarithmic plot of the HA-induced increase in sAP firing (HA effect) against basal sAP firing rate (HA effect vs. AP Basal R2 = 0.471). (O) Normalized time-course summary (left) and quantification (right) of sAP firing frequency during HA application in cetirizine or ranitidine (HA in ranitidine: 116.6% ± 7.67%, n = 7; HA inCTZ: 175.1% ± 22.08%, n = 8, p = .034). (P) Representative traces of CIN sAP firing in dimaprit or 2-PyEA (scale bars = 50 pA/3s). (Q) Normalized time-course summary (left) and quantification (right) of sAP firing frequency during dimaprit or 2-PyEA application (dimaprit: 420.8% ± 43.76%, n = 7; 2-PyEA: 135.7% ± 22.60%, n = 5, p = .005). Error bars indicate SEM. *p < .05. ACSF, artificial cerebrospinal fluid; AP, action potential; CIN, cholinergic interneuron; CTZ, cetirizine; HA, histamine; NAc, nucleus accumbens; PV-IN, parvalbumin-expressing fast-spiking interneuron; Ran., ranitidine; RMP, resting membrane potential; sAP, spontaneous AP; tdT, terminal deoxynucleotidyl transferase.
