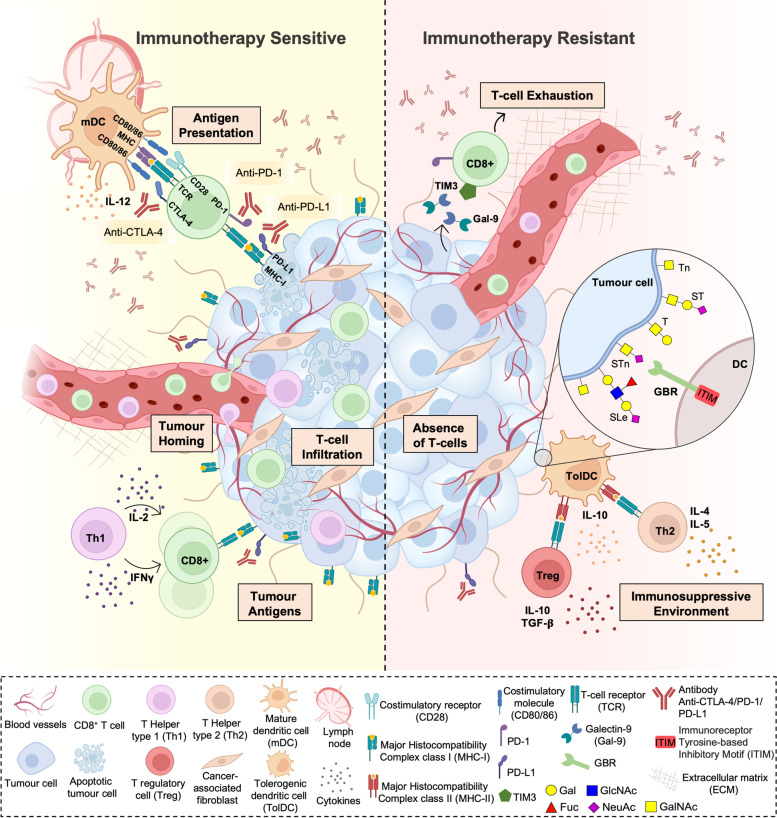
Fig. 1.
Currently accepted immune checkpoint inhibitors resistance mechanisms. Resistance to currently used immune checkpoint inhibitors, namely anti-PD1, anti-PD-L1 and anti-CTLA-4 therapeutic antibodies, has been associated with the lack of effective tumour T cell infiltration. This could be due to lack of tumour antigens, defects in antigen presentation, and absence of T cell activation. Of note, cancer-associated glycosignatures, (e.g. Tn, STn, lewis antigens, among others) that are distinct from those found on healthy cells, can interact with glycan-binding receptors (GBR) in immune cells, driving immunosuppression. Glycan-mediated immunosuppression is promoted by non-classical pathways, including altered antigen uptake, processing, and presentation by antigen presenting cells, ultimately conditioning T-cell priming. Moreover, GBRs engagement alters immune cell signalling, differentiation, and cytokine responses toward anti-inflammatory or immunosuppressive phenotypes. Furthermore, the abnormal glycosylation of tumour-associated glycoproteins generates neoantigens that can serve as targets for tumour-specific T cells