Fig. 2.
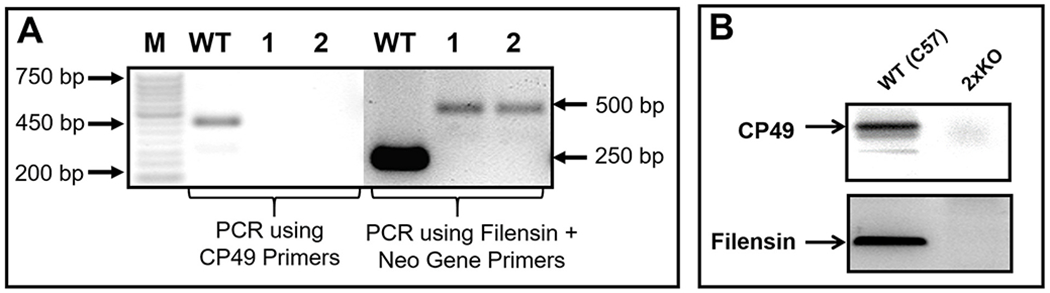
A. Genotyping. A double knockout mouse model, for lens-specific beaded filament proteins CP49 and filensin, was developed using CP49 (CP49-KO) and filensin (filensin-KO) knockout mice(Alizadeh et al., 2002, 2003), which do not have CP49 natural mutation. A Neomycin gene cassette was introduced during the process of single knockout mouse development ; genomic PCR was done to confirm the knockout of both proteins using the primer sets previously described by (Alizadeh et al., 2002, 2003). PCR of WT genomic DNA amplified a 450 bp amplicon denoting the presence of CP49. Lanes 1 and 2 ( samples from mouse 1 and mouse 2, respectively) representing samples for double knockout did not amplify the 450 bp fragment, confirming the disruption of the CP49 gene locus. With a mixture of filensin primers and Neo gene primers, WT genomic DNA amplified a 250 bp amplicon for the presence of filensin; the two double knockout samples (lanes marked as 1 and 2) amplified a 500 bp fragment (representative of the Neo gene cassette) confirming the disruption of the filensin gene locus. B. Western Blotting. WT mouse lens membrane proteins immunoreacted to CP49 and filensin antibodies indicating the expression of the respective proteins. 2xKO mouse lens membrane proteins showed no immunoreactivity to either antibody confirming the knockout status of CP49 and filensin gene loci.
