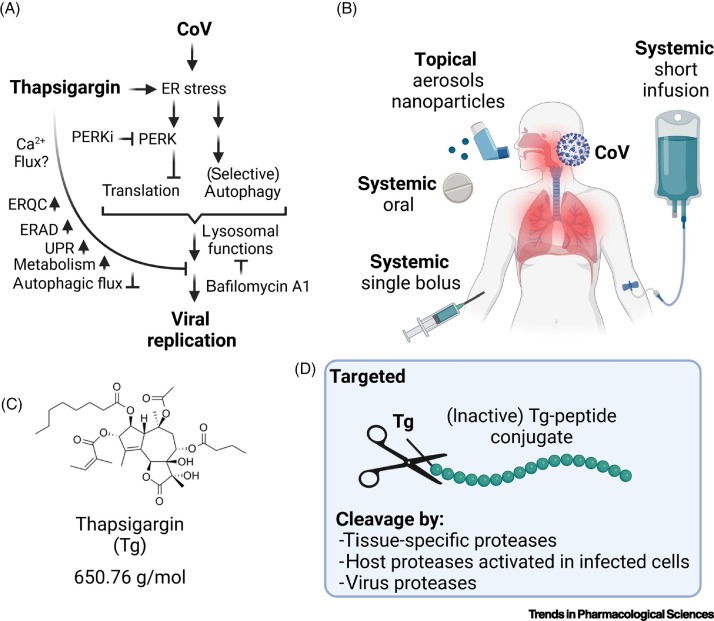
Figure 2.
Summary of thapsigargin effects in coronavirus (CoV)-infected cells and possible modes of application in humans.
(A) The scheme shows the different levels of cellular processes that have been shown to be activated or suppressed by thapsigargin in cells infected with various CoVs. Also included are the effects of drugs blocking the lysosome (bafilomycin A1) or PERK kinase (PERKi, GSK2656157 or GSK2606414). At present, the role of thapsigargin-mediated calcium depletion in coronavirus replication is unclear. Adapted from [15]. Abbreviations: Tg, thapsigargin; ER, endoplasmic reticulum; ERQC, ER quality control; ERAD, ER-associated protein degradation; PERK, protein kinase RNA-activated (PRKR)-like endoplasmic reticulum kinase; UPR, unfolded protein response. (B) Possible routes and modes of application of thapsigargin in humans. While short-term oral administration was effective in mice, the bioavailability and pharmacokinetics of thapsigargin for the use as antiviral therapy in humans are currently unclear. (C) Structure of thapsigargin. (D) Potential protease-cleavable prodrugs in which thapsigargin is coupled to a peptide (in green) that will be cleaved off by cell type-specific proteases, by (inducible) proteases present only in infected cells, or by coronavirus proteases, in order to minimize side effects by restricting the active drug primarily or exclusively to infected cells.