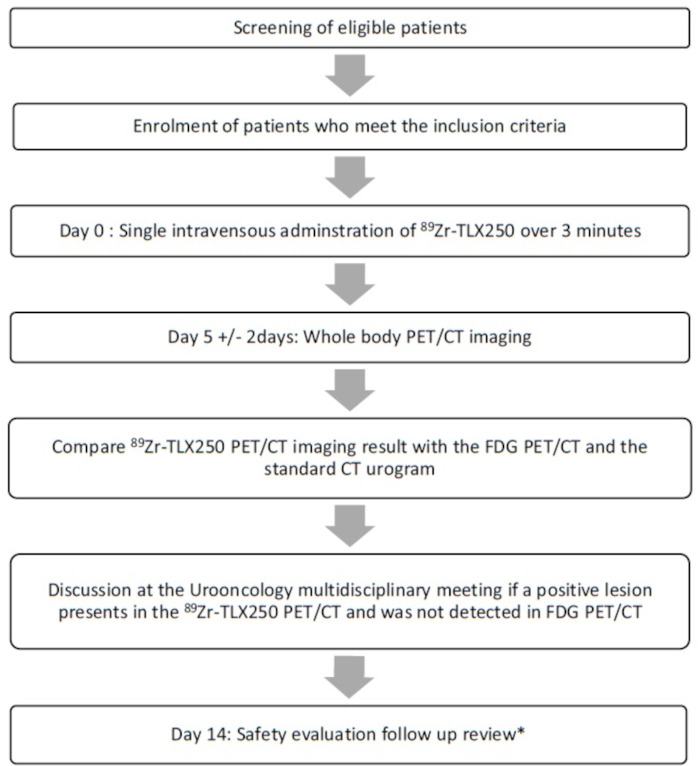
Figure 1.
Trial schema, schema showing the pathway for patients recruited into the phase I trial of 89Zirconium-labelled girentuximab (89Zr-TLX250) positron emitting tomography (PET) in 89Zirconium-labelled girentuximab PET in Urothelial Cancer Patients (ZiPUP). *Vital signs, standard laboratory (full blood count, renal function, basic electrolytes and liver function test), 12-lead ECG and concomitant medication recording and adverse event recording (NCI-CTCAE V.5.0). NCI-CTCAE V.5.0, National Cancer Institute-Common Terminology Criteria for Adverse Effects version V.5.0