Figure 2: EPCR presents late endosomal lysobisphosphatidic acid (LBPA) on the cell surface.
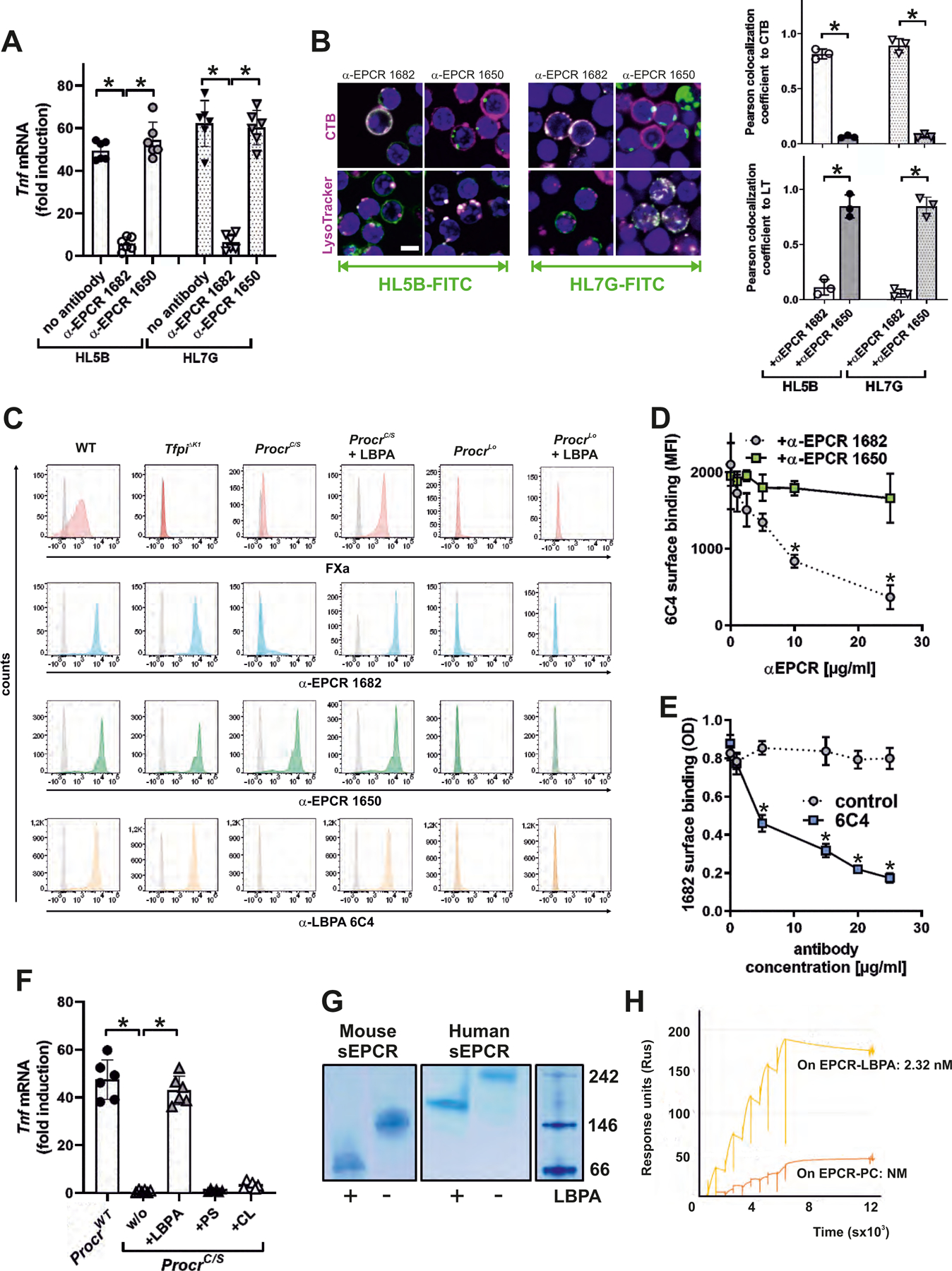
(A) Effect of blocking mouse EPCR with α-EPCR 1682 versus α-EPCR 1650 on Tnf mRNA induction after 3 hours of stimulation of mouse monocytes by aPL HL5B and HL7G; n=6, *P<0.0001; one-way ANOVA. (B) Effect of α-EPCR on aPL HL5B and HL7G (green) internalization in mouse CD115+ spleen monocytes with CTB or LysoTracker counterstaining (magenta). Bar=5 µm. Quantification of colocalization; n=3 ROI consisting of at least three cells, *P<0.0001; one-way ANOVA. (C) Flow cytometric detection of EPCR, LBPA, and FXa as marker for inhibited TF complex formation on CD115+ spleen monocytes isolated from the indicated mouse strains. Effect of pretreatment with 10 µM LBPA for 10 min on surface staining by the indicated antibodies in comparison to isotype control (gray). (D) Competition of α-EPCR 1650 and 1682 for binding of FITC-labeled α-LBPA 6C4 to mouse monocytes; n=3, *P≤0.001; two-way ANOVA, Sidak’s multiple comparisons test. (E) Competition of α-LBPA 6C4 for binding of α-EPCR 1682 to mouse monocytes; n=3, *P≤0.001; two-way ANOVA, Sidak’s multiple comparisons test. (F) Effect of pretreatment with LBPA, cardiolipin (CL), and phosphatidylserine (PS) (10 µM) on aPL HL5B signaling in ProcrC/S monocytes. Induction of Tnf mRNA after 3 hours is shown; n=6, *P<0.0001; one-way ANOVA. (G) LBPA loading of purified mouse or human sEPCR evidenced by faster mobility on native gels. (H) Surface plasmon resonance analysis of aPL HL5B binding to purified human sEPCR or sEPCR–LBPA. The affinity calculation was based on a monovalent binding model because no cooperative binding was evident. Affinity for EPCR with the typical structural lipid phosphatidyl choline (PC) (25) (EPCR-PC) was not measurable (NM).
