Scheme 1. Synthesis of Halo Analogues of 9 and Its Tritiated Form 18.
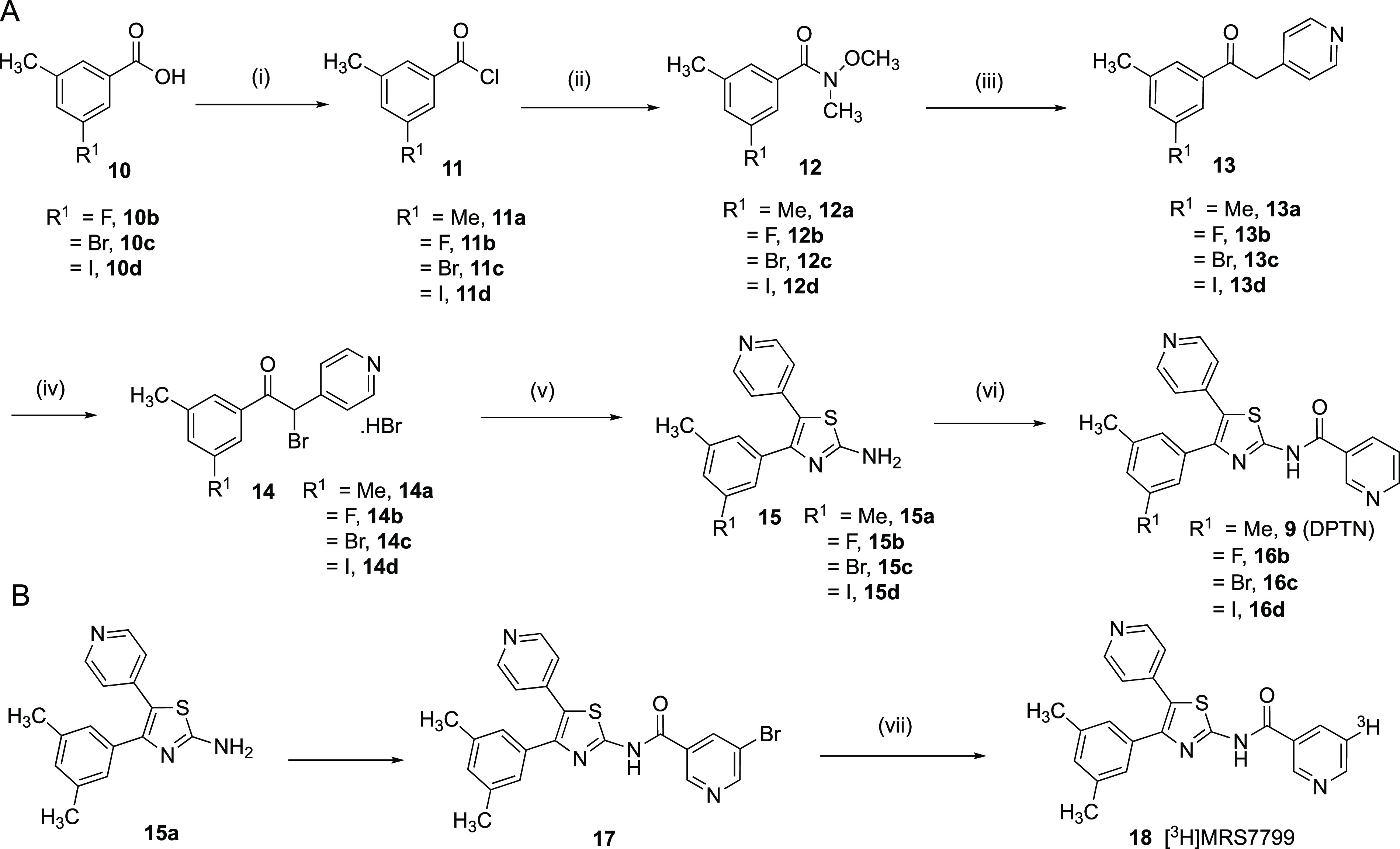
Reagents and conditions: (i) (COCl)2 (1.5 to 2.0 equiv), cat. DMF (10 μL), toluene, 0 °C to room temperature, 89–96%; (ii) N,O-dimethylhydroxylamine hydrochloride (1.2 to 1.5 equiv), K2CO3 (2.0 equiv), EtOAc–H2O (2:1), 0 °C to room temperature, 16–18 h, 86–98%; (iii) 4-picoline (1.2 to 1.5 equiv), LDA (1.0 M in THF/hexane (1.0–4.0 equiv), THF, −78 °C to room temperature, 2 to 3 h, 58–71%; (iv) Br2 (1.0 equiv), AcOH, 4 h, 80 °C, 43–90%; (v) methylthiourea (1.1 equiv), Et3N (2.1 to 3.0 equiv), ACN, reflux, 3 h, 38–87%; (vi) nicotinoyl chloride hydrochloride or 5-bromonicotinoyl chloride (1.5 equiv), DMAP (0.3 equiv) in DMA or NMP, 80 °C, 16 h; sat. NaHCO3, 39–87%; (vii) Pd/C, tritum gas.
