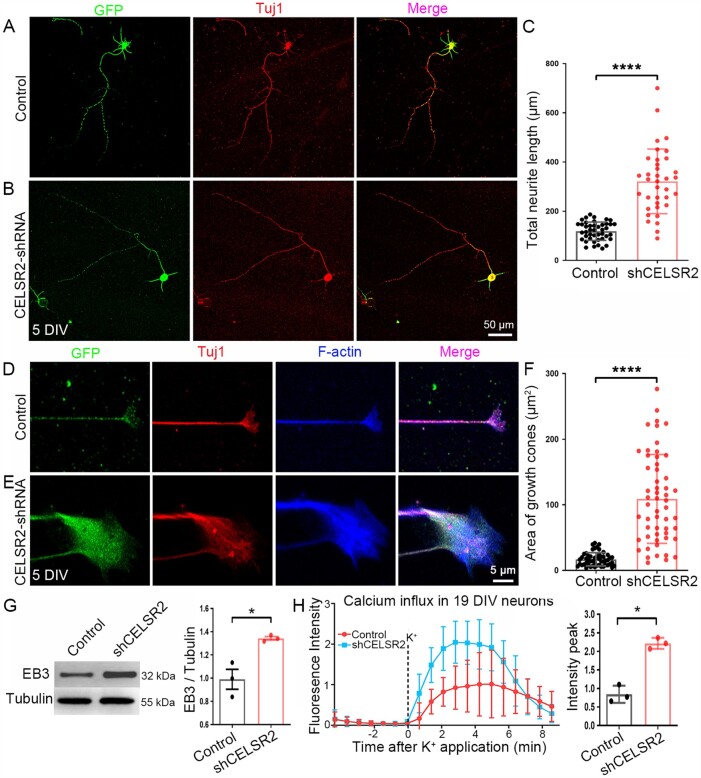
Figure 7.
CELSR2 knockdown promotes axonal growth in primary human spinal motor neuron culture. (A–C) CELSR2 scrambled shRNA (A, control) and CELSR2-shRNA (B) were used to transfect cultured primary spinal motor neurons from WPC7 and WPC8 human embryos. Transfected neurons were visualized by virus-encoded GFP (green). After 5 DIV, neurons were immunostained for Tuj1 (red). GFP- and Tuj1-immunoreactivity overlapped in the somas and neurites as shown in the merged images. A significant increase of total neurite length was observed in CELSR2-shRNA transfected neurons (C; in µm, control: 118.70 ± 5.66, CELSR2-shRNA: 321.28 ± 21.90, P < 0.0001, n = 43 in the control and n = 36 in the CELSR2-shRNA). (D–F) Double immunostaining for F-actin (blue) and Tuj1 (red) reveal axon shafts and growth cones in control (D) and CELSR2-shRNA (E) transfected neurons (GFP labelling, green). The growth cone area was significantly increased in CELSR2-shRNA versus control transfected neurons (F; control: 16.92 ± 1.40 µm2, and CELSR2-shRNA: 109.00 ± 9.12, P < 0.0001, n = 52 in the control and 55 in the CELSR2-shRNA). (G) Western blot analysis of 5-DIV cultured neurons with antibodies to EB3 and β-III tubulin (tubulin, reference) showed an increase of EB3 levels in CELSR2-shRNA transfected neurons (control: 0.99 ± 0.09, CELSR2-shRNA: 1.34 ± 0.02, P < 0.05, n = 3 independent experiments). (H) Intracellular calcium influx was evaluated in 19 DIV-cultured neurons by measuring Fluoro-4 AM fluorescence intensity. The curves were drawn from captured images before and after potassium application. There was an increase of the fluorescent peaks in CELSR2-shRNA transfected neurons (control: 0.84 ± 0.13, and CELSR2-shRNA: 2.22 ± 0.09, P < 0.05, n = 3 independent experiments). *P < 0.05; ****P < 0.0001; Student’s t-test.