Figure 4. Enduring functional significance of ELA-related microglial deficits.
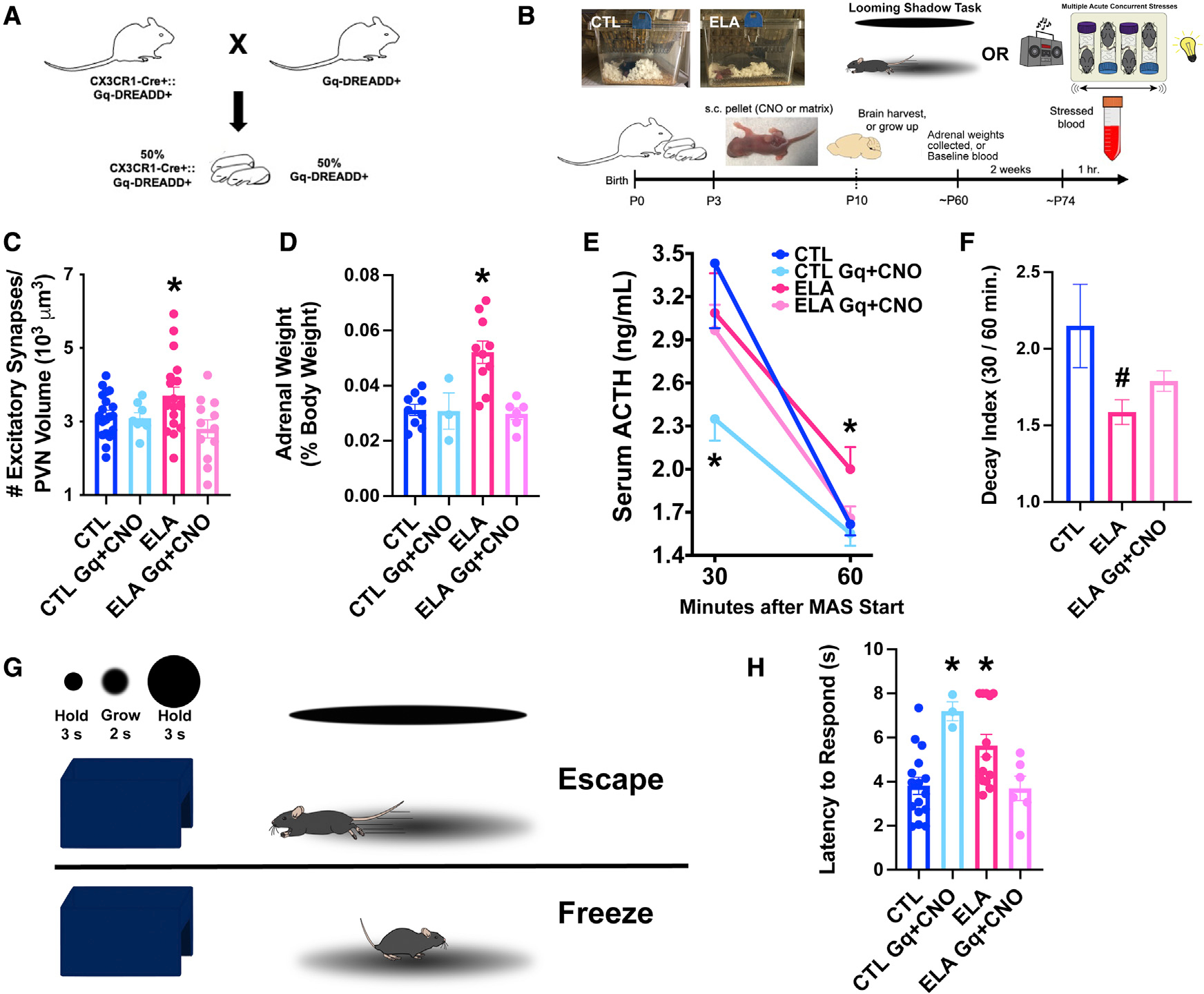
(A) Breeding strategy for the chemogenetic studies: CX3CR1-Cre+:Gq-DREADD+ mice were crossed with Gq-DREADD+ mice to generate ~50% pups expressing Gq-DREADDs exclusively in microglia (see Figures S4A and S4B).
(B) Schematic of in vivo chemogenetic activation experiment: Male mice of CX3CR1-Cre+:Gq-DREADD+ litters, randomly assigned to CTL or ELA conditions (P3) received small, sustained-release CNO- or placebo-containing pellets subcutaneously (s.c.). A cohort was perfused on P10 for the quantification of excitatory synapses on mpd-PVN cells. A second cohort were behaviorally tested as adults in the looming-shadow threat (Daviu et al., 2020) and provided adrenal gland weights, a measure of lifetime stress responses. A third cohort provided baseline and stress-induced ACTH and corticosterone (CORT) levels. After an adult acute, complex stress experience (Hokenson et al., 2020), blood was collected at 30 and 60 min from stress onset.
(C) Chronic chemogenetic microglial activation during P3–10 in microglia-specific Gq-DREADDs in ELA male mice decreased excitatory synapse numbers on mpd PVN neurons to control levels; this was not observed in mice lacking the microglial expression of Gq-DREADDs or in placebo-receiving mice (F2,23.8 = 3.76, p = 0.04; Welch’s 1-way ANOVA; p < 0.05, Dunnett’s T3 multiple comparisons test; also see Figure S6). As CNO alone did not alter the synapse number in microglial Gq-DREADD-expressing CTL mice (p > 0.6), CTL groups were combined for analysis.
(D) Adrenal weights of ELA-adult male mice were higher than those of control mice, indicative of lifelong exposure to augmented stress-hormone release. Chemogenetic microglial activation during the ELA epoch prevented adrenal weight increases (F3,24 = 11.11, p < 0.0001; 1-way ANOVA; p < 0.05, Holm-Sidak’s multiple comparisons test).
(E) At 30 min from onset, stress elicited a robust elevation of ACTH in both CTL and ELA male mice. Chemogenetic activation of microglia in CTL mice blunted this response (F3,20.6 = 3.19, p = 0.04; Welch’s 1-way ANOVA; p < 0.05, Dunnett’s T3 test). At 60 min from stress onset, ACTH levels dropped to 50% of peak values in CTL but not ELA mice (F2,53 = 3.45, p = 0.04; 1-way ANOVA; p < 0.05, Holm-Sidak’s test). Chemogenetic microglial activation in ELA mice ameliorated the prolonged surge of ACTH, which returned to control levels by 60 min (p > 0.8, Holm-Sidak’s test).
(F) The ACTH decay index (30 min/60 min levels) differed in ELA mice (F3,21.5 = 3.19, p = 0.04; Welch’s 1-way ANOVA; p = 0.06, Dunnett’s T3 test), and was partially restored in chemogenetically activated ELA mice (p > 0.2 compared to CTL, same post hoc test). The data show an aberrant prolongation of the hormonal stress response in ELA mice, and the restoration of normal “shutoff” mechanisms by early-life microglial activation (CTL Gq+ CNO mice were not included in the decay index analysis because their stress response was already blunted).
(G) Schematic of the looming-shadow threat task: mice experienced 5 looming-shadow stimulus trials, and their response was scored as escape (top), freezing (bottom), or none.
(H) ELA significantly prolonged latencies of responses to the threat (F3,35 = 6.25, p = 0.002; 1-way ANOVA; p < 0.05, Holm-Sidak’s test). Chemogenetic activation of microglia prevented ELA-induced aberrant threat responses (p > 0.9, Holm-Sidak’s test). Chemogenetic activation of microglia in CTL mice led to delayed responses to the threat, suggesting that normal responses require graded microglia-related synapse pruning (p < 0.05).
Means ± SEMs; *p < 0.05.
