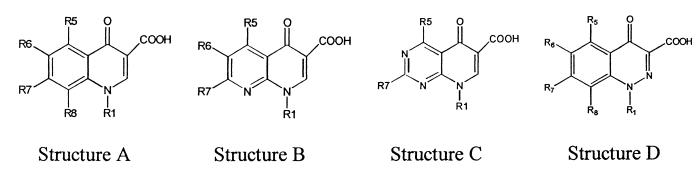
TABLE 4.
Structures of the quinolones tested and MICs
| Quinolone | Basea | Group atb:
|
Anti-MAI activity (μg/ml)c
|
|||||
|---|---|---|---|---|---|---|---|---|
| R1 | R8 | R5 | R6 | R7 | Exper. | Calc. | ||
| Moxifloxacin | A | Cyclopropyl | -OCH3 | H | F | Piperidino-pyrrolidinyl | 0.2 | 0.7 |
| Sparfloxacin | A | Cyclopropyl | F | NH2 | F | 3′,5′-Methylpiperazinyl | 0.4 | 0.3 |
| Gatifloxacin | A | Cyclopropyl | -OCH3 | H | F | 3′-Methylpiperazinyl | 0.9 | 0.8 |
| Temafloxacin | A | 2,4-Difluorophenyl | H | H | F | 3′-Methylpiperazinyl | 1.0 | 0.8 |
| Levofloxacin | A | H | F | 4′-Methylpiperazinyl | 2.1 | 4.0 | ||
| Ofloxacin | A | H | F | 4′-Methylpiperazinyl | 2.5 | 4.0 | ||
| Trovafloxacin | B | 2,4-Difluorophenyl | H | F | Aza-bicyclohexanyl | 2.7 | 1.7 | |
| Ciprofloxacin | A | Cyclopropyl | H | H | F | 1-Piperazinyl | 2.8 | 7.3 |
| Lomefloxacin | A | -CH2CH3 | F | H | F | 3′-Methylpiperazinyl | 4.5 | 4.9 |
| Clinafloxacin | A | Cyclopropyl | Cl | H | F | 3′-Aminopyrrolidinyl | 5.0 | 3.1 |
| Grepafloxacin | A | Cyclopropyl | H | CH3 | F | 3′-Methylpiperazinyl | 5.4 | 1.8 |
| Fleroxacin | A | -CH2CH2F | F | H | F | 4′-Methylpiperazinyl | 8.1 | 4.7 |
| Pefloxacin | A | -CH2CH3 | H | H | F | 4′-Methylpiperazinyl | 10.0 | 13.6 |
| Norfloxacin | A | -CH2CH3 | H | H | F | 1-Piperazinyl | 11.4 | 24.5 |
| Enoxacin | B | -CH2CH3 | H | F | 1-Piperazinyl | 13.7 | 27.7 | |
| Acrosoxacin | A | -CH2CH3 | H | H | H | 4-Pyridinyl | 23.5 | 68.8 |
| Rufloxacin | A | H | F | 1-Piperazinyl | 31.0 | 11.9 | ||
| Irloxacin | A | -CH2CH3 | H | H | F | 1-Pyrrolyl | 47.2 | 89.1 |
| Pipemidic acid | C | -CH2CH3 | H | 1-Piperazinyl | >250.0 | 95.8 | ||
| Flumequine | A | H | F | H | >250.0 | 82.1 | ||
| Piromidic acid | C | -CH2CH3 | H | 1-Pyrrolidinyl | >250.0 | 330.9 | ||
| Nalidixic acid | B | -CH2CH3 | H | H | CH3 | >250.0 | 462.5 | |
| Cinoxacin | D | -CH2CH3 | H | H | >250.0 | 486.3 | ||
| Oxolinic acid | A | -CH2CH3 | H | H | >250.0 | 449.3 | ||
A, B, C, and D, basic nucleus quinoline, 1,8-naphtyridine, pyrido[2,3-d]pyrimidine, and 1,2-cinnoline, respectively.
Levofloxacin R1 to R8, R1-CH(CH3)CH2O-R8; ofloxacin R1 to R8, R1-CH(CH3)CH2O-R8; rufloxacin R1 to R8, R1-CH2CH2S-R8; flumequine R1 to R8, R1-CH(CH3)CH2CH2-R8; cinoxacin R6 to R7, R6-O-CH2-O-R7; oxolinic acid R6 to R7, R6-O-CH2-O-R7.
Exper., experimental; calc, calculated.