Abstract
Single-brain neuroimaging studies have shown that human cooperation is associated with neural activity in frontal and temporoparietal regions. However, it remains unclear whether single-brain studies are informative about cooperation in real life, where people interact dynamically. Such dynamic interactions have become the focus of interbrain studies. An advantageous technique in this regard is functional near-infrared spectroscopy (fNIRS) because it is less susceptible to movement artifacts than more conventional techniques like electroencephalography (EEG) or functional magnetic resonance imaging (fMRI). We conducted a systematic review and the first quantitative meta-analysis of fNIRS hyperscanning of cooperation, based on thirteen studies with 890 human participants. Overall, the meta-analysis revealed evidence of statistically significant interbrain synchrony while people were cooperating, with large overall effect sizes in both frontal and temporoparietal areas. All thirteen studies observed significant interbrain synchrony in the prefrontal cortex (PFC), suggesting that this region is particularly relevant for cooperative behavior. The consistency in these findings is unlikely to be because of task-related activations, given that the relevant studies used diverse cooperation tasks. Together, the present findings support the importance of interbrain synchronization of frontal and temporoparietal regions in interpersonal cooperation. Moreover, the present article highlights the usefulness of meta-analyses as a tool for discerning patterns in interbrain dynamics.
Keywords: cooperation, fNIRS, hyperscanning, interbrain synchrony
Significance Statement
We present systematic review and the first quantitative meta-analysis of functional near-infrared spectroscopy (fNIRS) hyperscanning of cooperation, based on thirteen studies with 890 participants. All thirteen studies observed significant interbrain synchrony in the prefrontal cortex (PFC), suggesting that this region is particularly relevant for cooperative behavior. The present findings support the importance of interbrain synchronization of frontal and temporoparietal regions in interpersonal cooperation.
Introduction
Human beings cooperate on small scales, like friends or families, and on larger scales, like nation states (Jaeggi and Gurven, 2013; Handley and Mathew, 2020). Nevertheless, there are many cases where cooperation fails, from marital arguments to political conflicts, leading to suboptimal outcomes for individuals and society. To understand the complexities of cooperation and help people realize more of their cooperative potential, it is helpful to obtain a better scientific understanding of cooperation.
One key scientific question is how cooperation is implemented in the brain. Over the last three decades, a large literature has emerged on social neuroscience (Cacioppo et al., 2000; Todorov et al., 2011; Schurz et al., 2021). Much of this research to date has relied on a single-brain approach as the dominant paradigm in contemporary neuroscience. In a typical social neuroscience study, a participant views social stimuli on a computer screen while her or his neural activations are being recorded with electroencephalography (EEG) or functional magnetic resonance imaging (fMRI). A number of neural systems have been implicated in social cognition more generally, including the mirror neuron system and the mentalizing system. The former purportedly consists of the inferior frontal gyrus (IFG), inferior frontal lobule (IFL), and superior temporal gyrus (STG). The latter involves the temporoparietal junction (TPJ), precuneus, and prefrontal cortex (PFC; Rizzolatti and Fabbri-Destro, 2008; Van Overwalle and Baetens, 2009).
One limitation of traditional social neuroscience research is that participants are not directly engaged in social interaction. To overcome this problem, researchers have moved toward a truly social, second-person neuroscience approach (Schilbach et al., 2013; Redcay and Schilbach, 2019). In second-person neuroscience, neural processes are examined within the context of a real-time reciprocal social interaction. Preliminary evidence has confirmed the added value of the second-person neuroscience approach by showing that specific neural signatures are only observable during “true” social interaction (Tognoli et al., 2007).
Recent developments in neuroimaging have enabled so-called “hyperscanning,” whereby the activity of two or more brains can be assessed simultaneously while people are interacting (Dumas et al., 2010; Czeszumski et al., 2020). The resulting interbrain activity is usually characterized in terms of the synchronization of the functional activity of the interacting brains. Hyperscanning has used a variety of neural imaging procedures, including EEG (Goldstein et al., 2018), magnetoencephalography (MEG; Hirata et al., 2014), fMRI (Koike et al., 2016), and functional near-infrared spectroscopy (fNIRS; Scholkmann et al., 2013). Each apparatus and method has different advantages and disadvantages for hyperscanning (Czeszumski et al., 2020; Ayrolles et al., 2021). Hyperscanning research paradigms vary from studying coordinated finger movements (Tognoli et al., 2007), to real-life situations like playing guitar in a duet (Sänger et al., 2012) or studying multiple brains of high-school students inside the classroom (Dikker et al., 2017).
So far, hyperscanning studies have revealed that interbrain synchrony plays a crucial role in joint attention, interpersonal communication and coordination, cooperation, and decision-making (for review, see Czeszumski et al., 2020). Many hyperscanning studies have used spoken language during interactions between participants (Pérez et al., 2017; Kelsen et al., 2020; Z. Li et al., 2021), ranging from knowledge sharing, cooperation, turn-taking, and naturalistic situations. Of the latter studies, many reported the emergence of interbrain synchrony during interpersonal communication based on cooperative interaction in frontal and temporoparietal regions.
While the field is still young (Czeszumski et al., 2020), we conducted a meta-analysis (Zlowodzki et al., 2007) of fNIRS hyperscanning studies focusing on cooperative behavior. The present review focused explicitly on fNIRS studies for a number of reasons. The method of fNIRS is one of the most commonly used neuroimaging techniques in hyperscanning studies of cooperation (Kelsen et al., 2020), which is relatively insensitive to motion artifacts and capable of capturing interbrain synchrony over longer periods (from seconds to minutes).
For example, social communication enhanced interbrain synchrony during a turn-taking game (Nozawa et al., 2016). These and related findings suggest that interbrain synchrony in frontal regions is associated with successful knowledge sharing and cooperative behavior using spoken language. Studies have additionally reported higher interbrain synchrony in temporoparietal regions during teacher-student interactions (Zheng et al., 2018; Liu et al., 2019), cooperation (Xue et al., 2018; Lu et al., 2019a), and naturalistic discussion (Jiang et al., 2015).
In sum, many hyperscanning studies have examined the interbrain dynamics associated with cooperative behavior. The findings appear to show some convergence, with interbrain synchrony seemingly emerging in frontal regions. However, without quantitative integration through meta-analysis, it is not possible to determine the degree to which hyperscanning studies of cooperation have converging results. This question is of substantive theoretical interest, given the diverse paradigms used in hyperscanning studies in this area. More specifically, the cooperation tasks used varied considerably across studies, ranging from singing together to jointly solving a puzzle. This means that these tasks, aside from their cooperative nature, are unlikely to evoke shared neural activations based on low-level operational features. Thus, finding a common neuroanatomical site for interbrain synchrony in these studies would provide relatively strong evidence for a general-purpose neural substrate for cooperative behavior. Our work had two aims: (1) to review the relevant literature and (2) to assess consistency in findings of interbrain synchrony in different brain regions related to cooperative behavior.
Materials and Methods
Search strategy and inclusion criteria
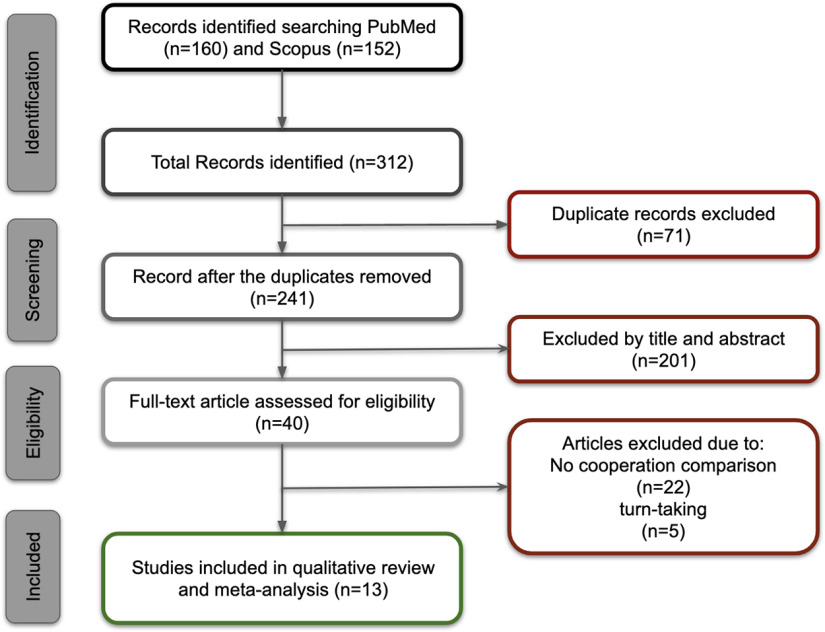
We searched MEDLINE and SCOPUS databases for fNIRS hyperscanning studies of cooperation in accordance with preferred reporting items for systematic reviews and meta-analysis guidelines (PRISMA; Moher et al., 2009). Following consultation with a librarian, two authors independently conducted searches in September 2021 using keywords: ((hyperscanning OR “social neuroscience” OR fnirs) AND (interbrain OR interbrain OR interpersonal OR interneural OR interneural OR synchron* OR coupling OR alignment OR “functional connectivity”) AND (cooperat* OR collaborat*)). Inclusion criteria included: fNIRS hyperscanning; cooperation/collaboration (where participants interacted to achieve a specific outcome such as solve a problem or puzzle or accomplish a particular result, thereby excluding turn-taking activities such as sequential counting, ultimatum game, prisoner dilemma and word games). Additionally, we excluded studies that focused on comparisons between genders, different levels of cooperation and did not report comparisons between cooperation and other conditions (cooperation or independent) or baseline. Discrepancies relating to inclusion were resolved through mutual discussion (Fig. 1).
Figure 1.
Flowchart of selection process.
Statistical analyses
Because functional equivalence was not expected to hold across the included studies, and a common effect size could not be assumed, we performed a random-effects meta-analysis (Borenstein et al., 2009). We set the threshold for Type I errors (α) at 0.05 and used effect sizes provided in the selected articles (if reported). We used the Psychometrica website (Lenhard and Lenhard, 2016) to estimate Cohen’s d from η2 (if available in the article), or we estimated Cohen’s d based on information provided in the article (statistical results; Lipsey and Wilson, 2001). Further, we transformed effect sizes to Hedges’ g; although similar to the classical Cohen’s d, it controls potential biases in studies with small sample sizes. If more than one comparison between cooperation and other conditions was present in the article, we chose the most orthogonal comparison. Furthermore, if more than one channel per region was reported, we selected the most central channel to the reported brain region. The heterogeneity across studies was gauged by Cochrane’s Q, I2, τ2 statistics, and forest plots. We used Cochrane’s Q as a statistical test of the null-hypothesis of no heterogeneity, I2 to quantitatively estimate the variance between studies, and forest plots to visualize all effect sizes. In addition, we used funnel plots to assess publication bias. Publication bias concerns the elevated probability of studies reporting positive results being published. The tendency of journals to give preference to research showing positive findings means negative results may remain unpublished, leading to bias and an increased likelihood of false-positive outcomes (Zlowodzki et al., 2007). Using Egger’s tests, we tested the funnel plot for symmetry and adjusted effect sizes with trim and fill analysis (Egger et al., 1997). Furthermore, we performed meta-regression analysis to test the influence of the variables Age, Gender and Language, type of communication on overall effect sizes. All statistics were computed using the open-source JASP statistical computing environment (JASP Team, 2020).
Results
We first present the results of the literature review and afterward the results of the meta-analysis of thirteen selected papers.
Selected studies
The search resulted in selecting thirteen studies over the period 2016–2021, with an initial total of 888 participants and 847 once unusable data were removed (see Table 1). Nine studies were conducted in China, one in Japan, and three were performed in the United States. Seven studies used verbal communication between acting participants during the investigation, while six studies did not. HbO measures were used because of increased sensitivity to blood flow, with preprocessing including low-pass filtering and global detrending. Eleven of the studies employed wavelet transform coherence (WTC; Grinsted et al., 2004) to convert the signal for interbrain synchrony analysis, and two studies used correlation-based measures to estimate interbrain synchrony.
Table 1.
Selected studies
| Study | Country Language |
Sample size# Relationship |
Age M |
SD | Activity | Oral communication |
Channels Phase analysis IBS regions |
IBS comparison |
|---|---|---|---|---|---|---|---|---|
| Liu et al. (2016) | • United States • English |
• 18 F-F = 2 F-M = 5 M-M = 2 • Strangers |
21.1 | 1.7 | Jenga game | Yes | • 19 • WTC • IFG/MFG |
Cooperation > dialogue |
| Fishburn et al. (2018) | • United States • English |
• 60 (57) F = 37 • Strangers |
19.73 | 1.02 | Tangram puzzle | Yes | • 18 spread over triad • Autoregressive model and robust correlation • IFG/MFG |
Together active > apart |
| Xue et al. (2018) | • China • Chinese |
• 90 (60) F = 43 • Strangers |
20 | 2.13 | Realistic presented problem |
Yes | • 46 • WTC • DLPFC and TPJ |
More cooperative dyads > less/no cooperative dyads |
| Lu and Hao (2019) | • China • Chinese |
• 44 (42) F = 40 • Strangers |
20.66 | 2.29 | Realistic presented problem |
Yes | • 22 • WTC • DLPFC |
Real participants > confederate |
| Lu et al. (2019a) | • China • Chinese |
• 118 F = 102 • Strangers |
20.72 | 2.47 | Realistic presented problem |
Yes | • 22 • WTC • FPC and DLPFC |
Positive and negative feedback > control |
| Lu et al. (2019b) | • China • Chinese |
• 104 (102) F = 64 • Strangers |
21 | 1.52 | Creativity task | Yes | • 46 • WTC • DLPFC and TPJ |
Cooperation > competition |
| Duan et al. (2020) | • China • Chinese |
• 84 F-M dyads • Lovers = 20 Strangers = 22 |
20.3 | 0.84 | Realistic presented problem |
Yes | • 19 • WTC • FPC, TPJ |
Lovers (cooperative) > strangers (no cooperative) |
| Sun et al. (2020) | • China • Chinese |
• 68 • 16 novice teachers (M = 3) • 18 expert teachers (M = 4) • 34 students (M = 7) • Same sex dyads • Strangers |
NT (25.81) ET (38.00) S (20.15) |
NT (4.69) ET (4.30) S (1.67) |
Math task | No | • 22 • WTC • DLPFC |
Cooperative > independent |
| Y. Li et al. (2021) | • China • Chinese |
• 90 (86) F = 45 M-M = 13 M- F = 15 F-F = 15. • Strangers |
21.14 | 2.01 | Jenga game | No | • 22 • WTC • IFG/MFG |
Cooperation > competition |
| Dai et al. (2018) | • China • Chinese |
• 84 • Same sex dyads • Strangers |
22.77 | 2.19 | Joint tapping task |
No | • 22 • Correlation • IFG/MFG |
Biderection > unidirectional |
| Osaka et al. (2015) | • Japan • Japanese |
• Singing 30 M-M = 8 F-F = 7 • Humming 28 M-M = 9 F-F = 5 • Stranger |
S (22) H (21) |
Missing | Singing | No | • 22 • WTC • IFG/MFG • Parietal cortex • MTG • IT |
Cooperative > alone |
| Li et al. (2020) | • China • Chinese |
• 48 • Familiar |
19.8 | 1.65 | Joint drawing task |
No | • 22 • WTC • DLPFC |
Cooperative > alone |
| Cui et al. (2012) | • United States • English |
• 22 F = 12 M-M = 1 M-F = 8 F-F = 2 |
26 | 6 | Joint tap | No | • 22 • WTC • SFG |
Cooperation > competition |
#Figures in parentheses = sample size after removing unused data; relationship = participants either known or unknown to each other; F = female; M = male; PFC = prefrontal cortex; MFG = middle frontal gyrus; IFG = inferior frontal gyrus; FPC = frontopolar cortex; DLPFC = dorsolateral PFC; SFG = superior frontal gyrus; TPJ = temporoparietal junction; MTG = middle temporal gyrus; IT = inferior temporal cortex; WTC = wavelet transform coherence.
Experimental designs
The conditions under which interbrain synchrony occurred depended on the experimental setup. Cooperative behavior is often studied with the use of games. Our search found three studies that used Jenga or Tangram puzzles to investigate interbrain synchrony (Jenga, Liu et al., 2016; Y. Li et al., 2021; Tangram, Fishburn et al., 2018). In the case of the Jenga game, these studies compared cooperative and competitive modes of building a tower, while solving a tangram puzzle was compared between together and apart conditions. On the one hand, multiple studies used different types of problem-solving tasks to study interbrain synchrony. A set of studies (Xue et al., 2018; Lu and Hao, 2019; Lu et al., 2019a; Duan et al., 2020) used realistically presented problem, where cooperation was facilitated by feedback and compared with situations where no feedback was provided. These studies used the presence of a third person (confederate) to create cooperative (feedback) and non-cooperative situations (no-feedback). This task closely resembles many everyday situations in which we solve problems together with the people surrounding us. They require communication and creativity; therefore, they are suitable for studying neural underpinnings of social interactions (interbrain synchrony).
Lu et al. (2019b) used a creativity task in cooperative and competitive contexts. Participants in this study had to solve problems that required divergent thinking. Another aspect of cooperation was studied with a math problem task by Sun et al. (2020) by comparing cooperative with independent situations between a teacher and student (both adults). On the other hand, tasks that cooperatively require synchronization of behavior were selected. Two studies investigated synchronized taps between participants. In one of them, participants tried to synchronize their taps (cooperation) or be faster than the co-actor (competition; Cui et al., 2012), while in the other study, bidirectional and unidirectional tapping was compared (Dai et al., 2018). Lastly, one study compared interbrain synchrony in joint (synchronized) versus independent drawing (Li et al., 2020). In sum, various types of tasks were found to study cooperation and interbrain synchrony with fNIRS. This suggests that many different cognitive functions were studied, and different brain regions were involved.
Brain regions
The results of the studies we reviewed showed interbrain synchrony in different parts of the brain. Studies reported parts of frontal and temporoparietal regions as sources of synchronization (Fig. 2).
Figure 2.
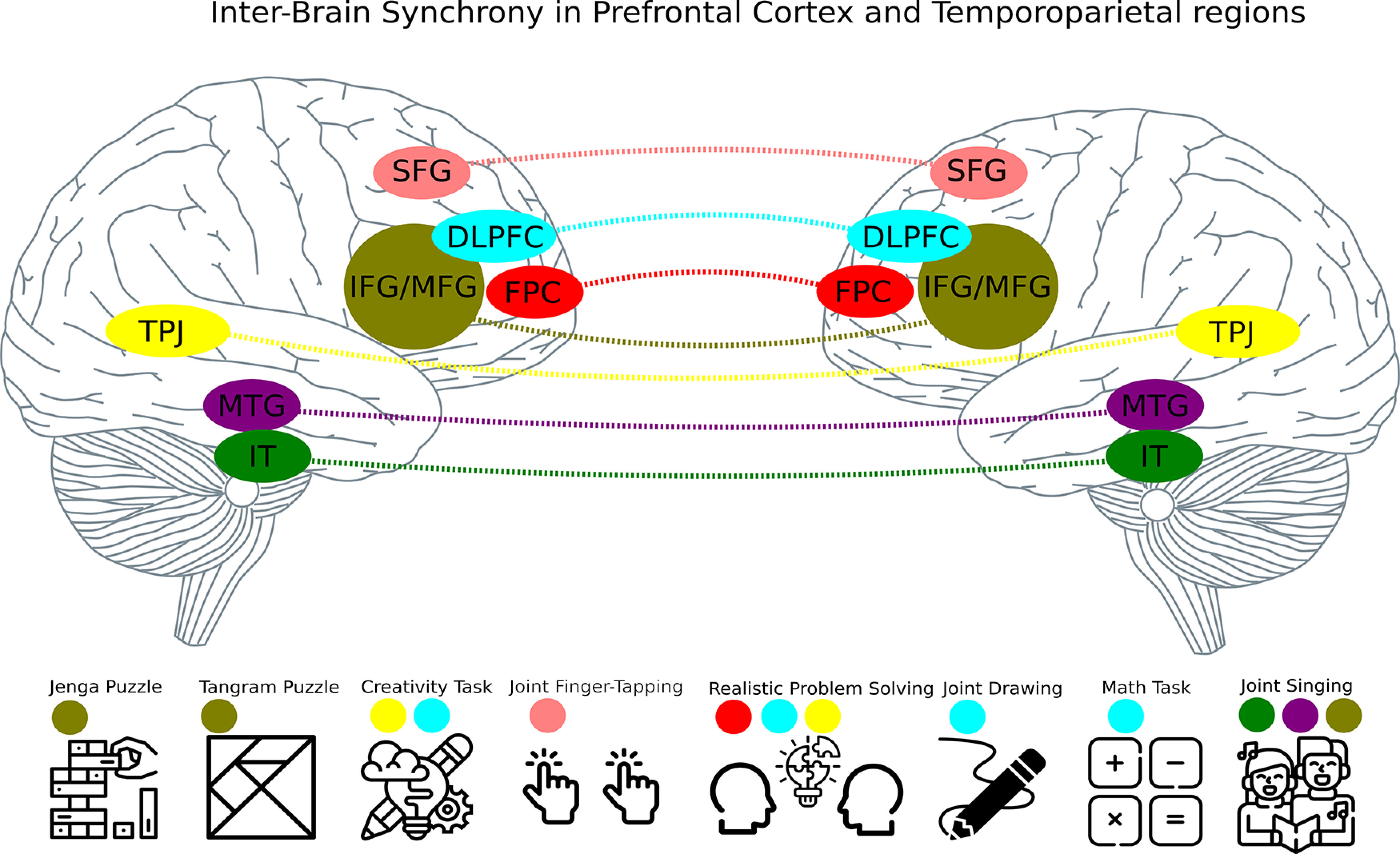
Interbrain synchrony in different parts of the prefrontal and temporoparietal cortex in various tasks used to study cooperation.
PFC
All studies report different subregions of PFC to elicit more robust interbrain synchrony in cooperative situations than the other conditions. Interestingly, different subparts of PFC were reported to be synchronized in different tasks. One set of studies (six studies, Xue et al., 2018; Lu and Hao, 2019; Lu et al., 2019a,b; Y. Li et al., 2020; Sun et al., 2020) that required flexibility in solving a problem (realistic, creativity, and math problems) or drawing together show interbrain synchrony in dorsolateral PFC (DLPFC). One of the primary functions of DLPFC reported in intra brain studies is cognitive flexibility related to attention switch (Monsell, 2003).
Collaborative problem-solving tasks require focus switches between co-actors and the problem to solve, and interbrain synchrony in DLPFC may underpin these flexible attentional switches. Different subregions of PFC, IFG/MFG, show interbrain synchrony during gamified tasks, like cooperative Jenga, tangram puzzle, and cooperative singing (four studies, Osaka et al., 2015; Liu et al., 2016; Fishburn et al., 2018; Y. Li et al., 2021). These regions are involved in language processing, and interbrain synchronization may facilitate cooperative behavior in tasks requiring a lot of verbal communication to solve (Jenga (with verbal communication) and Tangram puzzle; Liu et al., 2016; Fishburn et al., 2018). However, interbrain synchronization in IFG/MFG was also reported in cooperative Jenga play without verbal communication (Y. Li et al., 2021). Further research is needed to resolve the role of verbal communication in the Jenga task. One could compare cooperative Jenga play with and without verbal communication to gain more insight into the function of interbrain synchrony in IFG/MFG.
Another subpart of PFC that shows interbrain synchrony is SFG (superior frontal gyrus). We identified one experiment that showed higher interbrain synchrony for cooperative joint tap when compared with competitive (Cui et al., 2012). Lastly, we found that FPC (frontopolar cortex) also shows interbrain synchrony during cooperative realistic problem solving, suggesting that it is not only PFC that shows interbrain synchrony. Taken together, we found that most of the studies show interbrain synchrony in PFC, and that tasks requiring different cognitive functions elicit interbrain synchrony in different subparts of PFC.
Temporoparietal regions
Four of the included studies show interbrain synchrony in temporoparietal regions. It is important to note that these four studies are not different studies from the studies discussed above, but they show interbrain synchrony in temporoparietal regions in addition to PFC. Three out of four show interbrain synchrony in the TPJ while participants solve realistic or creativity problems (Xue et al., 2018; Lu et al., 2019b; Duan et al., 2020). TPJ is involved in many different tasks that require the theory of mind (Schurz et al., 2014), which is essential for successful interpersonal interactions as cooperative problem solving (Rilling et al., 2004). Therefore, the results of selected studies extend past research by showing interbrain synchrony in TPJ. Furthermore, these studies show interbrain synchrony in both frontal and temporoparietal regions, suggesting the existence of a PFC-TPJ interbrain network that facilitates cooperative behaviors. However, more evidence (studies) is required to test that interpretation. In addition to the PFC-TPJ connection, we identified one study that links PFC (IFG/MFG) with the temporal lobe (IT and MTG; inferior temporal cortex, middle temporal gyrus) during cooperative singing (Osaka et al., 2015).
Taken together, the selected studies pointed in the direction that interbrain synchrony in prefrontal and temporoparietal regions plays a crucial role in cooperation. To test that further, we performed a meta-analysis of the selected studies.
Meta-analysis
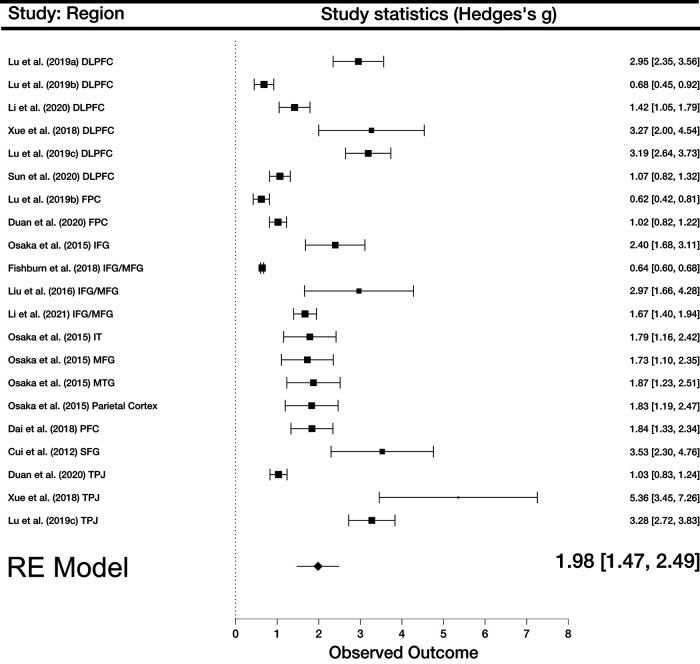
A random-effects model for all 21 experimental conditions across the thirteen studies reported a significantly large overall effect size (g = 1.98, 95% CI [1.47, 2.49], n = 21, z = 7.68, p < 0.001). Cochran’s Q statistic (Q = 469.72, p < 0.001) showed significant variation around the weighted average effect for the studies included. The proportion of observed variance was significantly high at I2 = 98.6 (>75 representing large heterogeneity), and a scaled measure of dispersion between true effect sizes of the studies was τ2 = 1.29 (Higgins and Thompson, 2002). These results suggest that the selected studies had an overall large effect size for comparison between cooperative and non-cooperative conditions. Furthermore, the variance between studies was high, suggesting that nearly all variance between studies was not because of chance. Visual inspection of the funnel plot and Egger’s test (z = 7.22, p < 0.001) indicated significant asymmetry. However, a follow-up trim and fill analysis resulted in the same effect size and confidence intervals (g = 1.98, 95% CI [1.47, 2.49]; Fig. 3).
Figure 3.
Forest plot of all included studies. Boxes represent effect sizes and whiskers confidence intervals.
We performed meta-regression examinations to test whether any independent variables (age, gender, language, type of communication) affected our analysis. Wald tests demonstrated no significant association between observed interbrain synchrony and independent variables overall. Chinese was used as the reference language. We found that age (β = 0.12, SE = 0.27, z = 0.43, p = 0.66), gender (β = −0.78, SE = 2.45, z = −0.32, p = 0.75), communication (β = 0.78, SE = 1.4, z = 0.56, p = 0.58), and language (English; β = 0.12, SE = 0.95, z = 0.12, p = 0.9 and Japanese; β = 0.29, SE = 0.92, z = 0.3, p = 0.77), all displayed insignificant results. The results of meta-regression analysis suggest that age, gender, type of communication, and language differences did not modulate overall effect sizes for the included studies.
Discussion
When people cooperate, their neural activity will tend to become mutually synchronized. This interbrain synchrony during cooperation tasks has become the focus of a growing number of hyperscanning studies. In the present article, we conducted a systematic review and meta-analysis of fNIRS hyperscanning studies of cooperation. We located thirteen relevant studies with a total of 890 participants. The results of our meta-analysis revealed significant overall effect sizes for interbrain synchrony in both frontal and temporoparietal regions. All studies observed significant interbrain synchrony in the PFC. This consistency is remarkable, considering that the included studies used various cooperation tasks, such as realistic problem solving, joint drawing, and the Jenga puzzle. It thus appears that PFC has general relevance for cooperative behavior that cannot be reduced to task-specific elements.
The findings of the present meta-analysis are broadly consistent with the findings of previous single-brain studies implicating prefrontal regions in tasks requiring social interaction, coordination, and cooperation (Stallen and Sanfey, 2013). The present findings not only confirm these earlier findings from single-brain recordings but show that they are part of a broader pattern indicating that prefrontal regions are not just activated within individual brains operating separately from another. Instead, prefrontal regions are mutually activated in a synchronized fashion in the brains of interaction partners, becoming coupled in their functioning. Hyperscanning studies thus complement and extend traditional social neuroscience studies that were conducted within the single-brain paradigm.
The present work has limitations. First, the present meta-analysis included a relatively low number of studies. The studies had a relatively high number of participants, which affords better statistical power. Still, the limited number of studies makes it hard to estimate the effects of between-study characteristics. Second, the present meta-analysis was restricted to a single neuroimaging method, fNIRS, which has limited spatial resolution. In the same line, the placement of recording channels is not standardized; therefore, it is difficult to compare different studies. It hence remains essential to compare the present findings to other neuroimaging methods, like fMRI. Third, the meta-analysis revealed a high variance between studies that cannot be explained by chance. More work is needed to understand the sources of this variance, which is likely because of the large variety of conditions used in different studies. Fourth and last, the present meta-analysis may be contaminated by reporting bias, given that published studies tend to report only statistically significant comparisons of neural recordings. It is important to note that the last limitation is not a limitation per se of our work but a more general limitation of many neuroimaging studies that the field should address. We propose that non-significant channels/comparisons should be reported in supplementary materials with all statistics values. It will allow for collecting more evidence and improve future meta-analyses. Additionally, this problem may be overcome in future work by creating better infrastructures for data sharing and open science practices (Pavlov et al., 2021).
In conclusion, human beings are a cooperative species. The present research uncovered some of the neural foundations of this human ability to cooperate by conducting the first systematic review and quantitative meta-analysis of fNIRS hyperscanning of cooperative behavior. The results showed that cooperation is consistently associated with interbrain synchrony in frontal and temporoparietal areas, suggesting that interbrain neural alignment in these regions underlies cooperative behavior in humans. These findings underscore the importance of meta-analyses in detecting patterns across studies and elucidating the neural basis of semi-naturalistic cooperative behavior.
Synthesis
Reviewing Editor: Macià Buades-Rotger, University of Luebeck
Decisions are customarily a result of the Reviewing Editor and the peer reviewers coming together and discussing their recommendations until a consensus is reached. When revisions are invited, a fact-based synthesis statement explaining their decision and outlining what is needed to prepare a revision will be listed below. The following reviewer(s) agreed to reveal their identity: Leonhard Schilbach, Edda Bilek.
Dear authors,
Thank you for your work and patience. Both reviewers and I are satisfied with the implemented changes, particularly the inclusion of more studies and the greater detail of the Introduction and Discussion. The article is now substantially more informative and will constitute a fine addition to the literature. I only have one small but important suggestion that should be corrected when proofreading the manuscript before final publication, namely:
- Page 11: “We propose that no significant channels/comparisons” -> We propose that *non-significant* channels...
Thanks again and congratulations.
References
- Ayrolles A, Brun F, Chen P, Djalovski A, Beauxis Y, Delorme R, Bourgeron T, Dikker S, Dumas G (2021) HyPyP: a hyperscanning Python pipeline for inter-brain connectivity analysis. Soc Cogn Affect Neurosci 16:72–83. 10.1093/scan/nsaa141 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borenstein M, Hedges LV, Higgins JPT, Rothstein HR (2009) Converting among effect sizes. In: Introduction to meta-analysis, Chapter 7, pp 45–49. Hoboken: Wiley, Ltd. [Google Scholar]
- Cacioppo JT, Berntson GG, Sheridan JF, McClintock MK (2000) Multilevel integrative analyses of human behavior: social neuroscience and the complementing nature of social and biological approaches. Psychol Bull 126:829–843. [DOI] [PubMed] [Google Scholar]
- Cui X, Bryant DM, Reiss AL (2012) NIRS-based hyperscanning reveals increased interpersonal coherence in superior frontal cortex during cooperation. Neuroimage 59:2430–2437. 10.1016/j.neuroimage.2011.09.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Czeszumski A, Eustergerling S, Lang A, Menrath D, Gerstenberger M, Schuberth S, Schreiber F, Rendon ZZ, König P (2020) Hyperscanning: a valid method to study neural inter-brain underpinnings of social interaction. Front Hum Neurosci 14:39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dai R, Liu R, Liu T, Zhang Z, Xiao X, Sun P, Yu X, Wang D, Zhu C (2018) Holistic cognitive and neural processes: a fNIRS-hyperscanning study on interpersonal sensorimotor synchronization. Soc Cogn Affect Neurosci 13:1141–1154. 10.1093/scan/nsy090 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dikker S, Wan L, Davidesco I, Kaggen L, Oostrik M, McClintock J, Rowland J, Michalareas G, Van Bavel JJ, Ding M, Poeppel D (2017) Brain-to-brain synchrony tracks real-world dynamic group interactions in the classroom. Curr Biol 27:1375–1380. [DOI] [PubMed] [Google Scholar]
- Dumas G, Nadel J, Soussignan R, Martinerie J, Garnero L (2010) Inter-brain synchronization during social interaction. PLoS One 5:e12166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duan H, Yang T, Wang X, Kan Y, Zhao H, Li Y, Hu W (2020) Is the creativity of lovers better? A behavioral and functional near-infrared spectroscopy hyperscanning study. Curr Psychol 41:41–54. 10.1007/s12144-020-01093-5 [DOI] [Google Scholar]
- Egger M, Smith GD, Schneider M, Minder C (1997) Bias in meta-analysis detected by a simple, graphical test. BMJ 315:629–634. 10.1136/bmj.315.7109.629 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fishburn FA, Murty VP, Hlutkowsky CO, MacGillivray CE, Bemis LM, Murphy ME, Huppert TJ, Perlman SB (2018) Putting our heads together: interpersonal neural synchronization as a biological mechanism for shared intentionality. Soc Cogn Affect Neurosci 13:841–849. 10.1093/scan/nsy060 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grinsted A, Moore JC, Jevrejeva S (2004) Application of the cross wavelet transform and wavelet coherence to geophysical time series. Nonlin Processes Geophys 11:561–566. 10.5194/npg-11-561-2004 [DOI] [Google Scholar]
- Goldstein P, Weissman-Fogel I, Dumas G, Shamay-Tsoory SG (2018) Brain-to-brain coupling during handholding is associated with pain reduction. Proc Natl Acad Sci U S A 115:E2528–E2537. 10.1073/pnas.1703643115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Handley C, Mathew S (2020) Human large-scale cooperation as a product of competition between cultural groups. Nat Commun 11:1–9. 10.1038/s41467-020-14416-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirata M, Ikeda T, Kikuchi M, Kimura T, Hiraishi H, Yoshimura Y, Asada M (2014) Hyperscanning MEG for understanding mother-child cerebral interactions. Front Hum Neurosci 8:118. 10.3389/fnhum.2014.00118 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Higgins JP, Thompson SG (2002) Quantifying heterogeneity in a meta‐analysis. Stat Med 21:1539–1558. 10.1002/sim.1186 [DOI] [PubMed] [Google Scholar]
- Jaeggi AV, Gurven M (2013) Natural cooperators: food sharing in humans and other primates. Evol Anthropol 22:186–195. 10.1002/evan.21364 [DOI] [PubMed] [Google Scholar]
- JASP Team (2020) JASP (version 0.14.1) [computer software]. Available at https://jasp-stats.org/.
- Jiang J, Chen C, Dai B, Shi G, Ding G, Liu L, Lu C (2015) Leader emergence through interpersonal neural synchronization. Proc Natl Acad Sci U S A 112:4274–4279. 10.1073/pnas.1422930112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelsen BA, Sumich A, Kasabov N, Liang SH, Wang GY (2020) What has social neuroscience learned from hyperscanning studies of spoken communication? A systematic review. Neurosci Biobehav Rev 132:1249–1262. [DOI] [PubMed] [Google Scholar]
- Koike T, Tanabe HC, Okazaki S, Nakagawa E, Sasaki AT, Shimada K, Sugawara SK, Takahashi HK, Yoshihara K, Bosch-Bayard J, Sadato N (2016) Neural substrates of shared attention as social memory: a hyperscanning functional magnetic resonance imaging study. Neuroimage 125:401–412. 10.1016/j.neuroimage.2015.09.076 [DOI] [PubMed] [Google Scholar]
- Lenhard W, Lenhard A (2016) Calculation of effect sizes. Available at https://www.psychometrica.de/effect_size.html. Dettelbach: Psychometrica. [Google Scholar]
- Li L, Wang H, Luo H, Zhang X, Zhang R, Li X (2020) Interpersonal neural synchronization during cooperative behavior of basketball players: a fNIRS-based hyperscanning study. Front Hum Neurosci 14:169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y, Chen R, Turel O, Feng T, Zhu CZ, He Q (2021) Dyad sex composition effect on inter-brain synchronization in face-to-face cooperation. Brain Imaging Behav 15:1667–1675. [DOI] [PubMed] [Google Scholar]
- Li Z, Li J, Hong B, Nolte G, Engel AK, Zhang D (2021) Speaker–listener neural coupling reveals an adaptive mechanism for speech comprehension in a noisy environment. Cereb Cortex 31:4719–4729. 10.1093/cercor/bhab118 [DOI] [PubMed] [Google Scholar]
- Lipsey MW, Wilson DB (2001) Practical meta-analysis. Thousand Oaks: SAGE Publications, Inc. [Google Scholar]
- Liu N, Mok C, Witt EE, Pradhan AH, Chen JE, Reiss AL (2016) NIRS-based hyperscanning reveals inter-brain neural synchronization during cooperative Jenga game with face-to-face communication. Front Hum Neurosci 10:82. 10.3389/fnhum.2016.00082 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu J, Zhang R, Geng B, Zhang T, Yuan D, Otani S, Li X (2019) Interplay between prior knowledge and communication mode on teaching effectiveness: interpersonal neural synchronization as a neural marker. Neuroimage 193:93–102. 10.1016/j.neuroimage.2019.03.004 [DOI] [PubMed] [Google Scholar]
- Lu K, Hao N (2019) When do we fall in neural synchrony with others? Soc Cogn Affect Neurosci 14:253–261. 10.1093/scan/nsz012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu K, Qiao X, Hao N (2019a) Praising or keeping silent on partner’s ideas: leading brainstorming in particular ways. Neuropsychologia 124:19–30. 10.1016/j.neuropsychologia.2019.01.004 [DOI] [PubMed] [Google Scholar]
- Lu K, Xue H, Nozawa T, Hao N (2019b) Cooperation makes a group be more creative. Cereb Cortex 29:3457–3470. 10.1093/cercor/bhy215 [DOI] [PubMed] [Google Scholar]
- Moher D, Liberati A, Tetzlaff J, Altman DG; PRISMA Group (2009) Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. Ann Intern Med 151:264–269. 10.7326/0003-4819-151-4-200908180-00135 [DOI] [PubMed] [Google Scholar]
- Monsell S (2003) Task switching. Trends Cogn Sci 7:134–140. 10.1016/S1364-6613(03)00028-7 [DOI] [PubMed] [Google Scholar]
- Nozawa T, Sasaki Y, Sakaki K, Yokoyama R, Kawashima R (2016) Interpersonal frontopolar neural synchronization in group communication: an exploration toward fNIRS hyperscanning of natural interactions. Neuroimage 133:484–497. 10.1016/j.neuroimage.2016.03.059 [DOI] [PubMed] [Google Scholar]
- Osaka N, Minamoto T, Yaoi K, Azuma M, Shimada YM, Osaka M (2015) How two brains make one synchronized mind in the inferior frontal cortex: fNIRS-based hyperscanning during cooperative singing. Front Psychol 6:1811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pavlov YG, Adamian N, Appelhoff S, Arvaneh M, Benwell CSY, Beste C, Bland AR, Bradford DE, Bublatzky F, Busch NA, Clayson PE, Cruse D, Czeszumski A, Dreber A, Dumas G, Ehinger B, Ganis G, He X, Hinojosa JA, Huber-Huber C, et al. (2021) #EEGManyLabs: investigating the replicability of influential EEG experiments. Cortex 144:213–229. [DOI] [PubMed] [Google Scholar]
- Pérez A, Carreiras M, Duñabeitia JA (2017) Brain-to-brain entrainment: EEG interbrain synchronization while speaking and listening. Sci Rep 7:4190. 10.1038/s41598-017-04464-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Redcay E, Schilbach L (2019) Using second-person neuroscience to elucidate the mechanisms of social interaction. Nat Rev Neurosci 20:495–505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rilling JK, Sanfey AG, Aronson JA, Nystrom LE, Cohen JD (2004) The neural correlates of theory of mind within interpersonal interactions. Neuroimage 22:1694–1703. [DOI] [PubMed] [Google Scholar]
- Rizzolatti G, Fabbri-Destro M (2008) The mirror system and its role in social cognition. Curr Opin Neurobiol 18:179–184. [DOI] [PubMed] [Google Scholar]
- Sänger J, Müller V, Lindenberger U (2012) Intra- and interbrain synchronization and network properties when playing guitar in duets. Front Hum Neurosci 6:312. 10.3389/fnhum.2012.00312 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schilbach L, Timmermans B, Reddy V, Costall A, Bente G, Schlicht T, Vogeley K (2013) Toward a second-person neuroscience 1. Behav Brain Sci 36:393–414. 10.1017/S0140525X12000660 [DOI] [PubMed] [Google Scholar]
- Scholkmann F, Holper L, Wolf U, Wolf M (2013) A new methodical approach in neuroscience: assessing inter-personal brain coupling using functional near-infrared imaging (fNIRI) hyperscanning. Front Hum Neurosci 7:813. 10.3389/fnhum.2013.00813 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schurz M, Radua J, Aichhorn M, Richlan F, Perner J (2014) Fractionating theory of mind: a meta-analysis of functional brain imaging studies. Neurosci Biobehav Rev 42:9–34. 10.1016/j.neubiorev.2014.01.009 [DOI] [PubMed] [Google Scholar]
- Schurz M, Radua J, Tholen MG, Maliske L, Margulies DS, Mars RB, Sallet J, Kanske P (2021) Toward a hierarchical model of social cognition: a neuroimaging meta-analysis and integrative review of empathy and theory of mind. Psychol Bull 147:293–327. 10.1037/bul0000303 [DOI] [PubMed] [Google Scholar]
- Stallen M, Sanfey AG (2013) The cooperative brain. Neuroscientist 19:292–303. 10.1177/1073858412469728 [DOI] [PubMed] [Google Scholar]
- Sun B, Xiao W, Feng X, Shao Y, Zhang W, Li W (2020) Behavioral and brain synchronization differences between expert and novice teachers when collaborating with students. Brain Cogn 139:105513. [DOI] [PubMed] [Google Scholar]
- Todorov A, Fiske S, Prentice D, eds (2011) Social neuroscience: toward understanding the underpinnings of the social mind. Oxford: Oxford University Press. [Google Scholar]
- Tognoli E, Lagarde J, DeGuzman GC, Kelso JS (2007) The phi complex as a neuromarker of human social coordination. Proc Natl Acad Sci U S A 104:8190–8195. 10.1073/pnas.0611453104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Overwalle F, Baetens K (2009) Understanding others’ actions and goals by mirror and mentalizing systems: a meta-analysis. Neuroimage 48:564–584. [DOI] [PubMed] [Google Scholar]
- Xue H, Lu K, Hao N (2018) Cooperation makes two less-creative individuals turn into a highly-creative pair. Neuroimage 172:527–537. 10.1016/j.neuroimage.2018.02.007 [DOI] [PubMed] [Google Scholar]
- Zheng L, Chen C, Liu W, Long Y, Zhao H, Bai X, Zhang Z, Han Z, Liu L, Guo T, Chen B, Ding G, Lu C (2018) Enhancement of teaching outcome through neural prediction of the students’ knowledge state. Hum Brain Mapp 39:3046–3057. 10.1002/hbm.24059 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zlowodzki M, Poolman RW, Kerkhoffs GM, Tornetta P 3rd, Bhandari M; International Evidence-Based Orthopedic Surgery Working Group (2007) How to interpret a meta-analysis and judge its value as a guide for clinical practice. Acta Orthop 78:598–609. 10.1080/17453670710014284 [DOI] [PubMed] [Google Scholar]