TABLE 2.
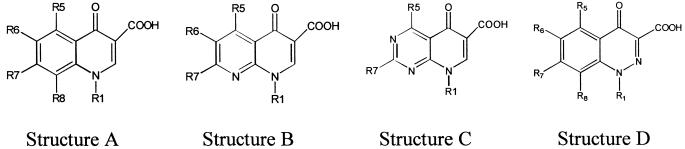
Structures of the 24 quinolones studied, in order of decreasing experimental and calculated IC50s against T. gondii
| Quinolone | Basea | R1 | R8 | R5 | R6 | R7 | IC50 (mg/liter)
|
|
|---|---|---|---|---|---|---|---|---|
| Exptl | Calculated | |||||||
| Trovafloxacin | B | 2,4-Difluorophenyl | H | F | Azabicyclohexane | 0.4 | 0.5 | |
| Grepafloxacin | A | Cyclopropyl | H | CH3 | F | 3′-Methylpiperazine | 2.4 | 4.4 |
| Gatifloxacin | A | Cyclopropyl | −OCH3 | H | F | 3′-Methylpiperazine | 4.1 | 9.0 |
| Moxifloxacin | A | Cyclopropyl | −OCH3 | H | F | Piperidinopyrrolidine | 5.1 | 2.5 |
| Temafloxacin | A | 2,4-Difluorophenyl | H | H | F | 3′-Methylpiperazine | 11.5 | 17.3 |
| Clinafloxacin | A | Cyclopropyl | Cl | H | F | 3′-Aminopyrrolidine | 15.0 | 15.6 |
| Acrosoxacin | A | −CH2CH3 | H | H | H | 4′-Pyridine | 20.3 | 27.9 |
| Enoxacin | B | −CH2CH3 | H | F | Piperazine | 20.3 | 26.1 | |
| Lomefloxacin | A | −CH2CH3 | F | H | F | 3′-Methylpiperazine | 21.2 | 21.2 |
| Rufloxacin | A | —b | —b | H | F | Piperazine | 22.3 | 20.6 |
| Irloxacin | A | −CH2CH3 | H | H | F | Pyrrole | 22.4 | 24.2 |
| Piromidic acid | C | −CH2CH3 | H | Pyrrolidine | 26.2 | 40.7 | ||
| Sparfloxacin | A | Cyclopropyl | F | NH2 | F | 3′,5′-Methylpiperazine | 39.5 | 20.1 |
| Flumequin | A | —c | —c | H | F | H | 40.6 | 43.8 |
| Fleroxacin | A | −CH2CH2F | F | H | F | 4′-Methylpiperazine | 46.8 | 44.6 |
| Oxolinic acid | A | −CH2CH3 | H | H | —d | —d | 47.2 | 46.5 |
| Norfloxacin | A | −CH2CH3 | H | H | F | Piperazine | 48.3 | 47.7 |
| Ofloxacin | A | —e | —e | H | F | 4′-Methylpiperazine | 53.6 | NCf |
| Nalidixic acid | B | −CH2CH3 | H | H | CH3 | 73.6 | 68.7 | |
| Ciprofloxacin | A | Cyclopropyl | H | H | F | Piperazine | 79.4 | 27.9 |
| Pefloxacin | A | −CH2CH3 | H | H | F | 4′-Methylpiperazine | 77.7 | 141.7 |
| Pipemidic acid | C | −CH2CH3 | H | Piperazine | 116.4 | 39.3 | ||
| Levofloxacin | A | —e | —e | H | F | 4′-Methylpiperazine | 159.6 | NC |
| Cinoxacin | D | −CH2CH3 | H | H | —d | —d | 200.0 | NC |
| K1g | B | 2,4-Difluorophenyl | H | F | 2-Methyl-6-amino-3aza-bicyclo[3.1.0.]hexyl | 0.2 | 0.2 | |
| K2 | B | 2,4-Difluorophenyl | H | F | 6-Aminomethyl-3aza-bicyclo[3.1.0.]hexyl | 0.2 | 0.6 | |
| K3 | B | 2,4-Difluorophenyl | CH3 | F | 6-Amino-3-azabicyclo[3.1.0.]hexyl | 0.3 | 0.1 | |
| K4 | B | Cyclopropyl | H | F | 6-Amino-3-azabicyclo[3.1.0.]hexyl | 0.6 | 0.8 | |
| K5 | B | 2,4-Difluorophenyl | H | F | 6-Amino-3-azabicyclo[3.1.0.]hexyl | 0.9 | 0.4 | |
| K6 | A | 2,4-Difluorophenyl | H | H | F | 6-Amino-3-azabicyclo[3.1.0.]hexyl | 1.1 | 0.7 |
| K7 | B | 2,4-Difluorophenyl | H | F | 2-Methyl-6-aminomethyl-3aza-bicyclo[3.1.0.]hexyl | 1.5 | 0.2 | |
| K8 | B | 2,4-Difluorophenyl | H | F | 5-Amino-3-azabicyclo[3.1.0.]hexyl | 3.0 | 5.2 | |
| K9 | B | Cyclopropyl | H | F | 6-Methylamino-3aza-bicyclo[3.1.0.]hexyl | 4.3 | 1.0 | |
| K10 | B | Cyclopropyl | H | F | 5-Methylamino-3-azabicyclo[3.1.0.]hexyl | 4.5 | 12.1 | |
| K11 | B | 2,4-Difluorophenyl | H | F | 6-Methylamino-3aza-bicyclo[3.1.0.]hexyl | 4.3 | 0.5 | |
Structures A, B, C, and D represent the basic quinoline nucleus, 1,8-naphthyridine, pyrido[2,3-d]pyrimidine, and 1,2-cinnoline, respectively.
R1-CH2CH2S-R8.
R1-CH(CH3)CH2CH2-R8.
R6-O-CH2-O-R7.
R1-CH(CH3)CH2-O-R8.
NC, not calculated.
Quinolone structures K1 to K11 and IC50s were described by Khan et al. (10).