FIG 1.
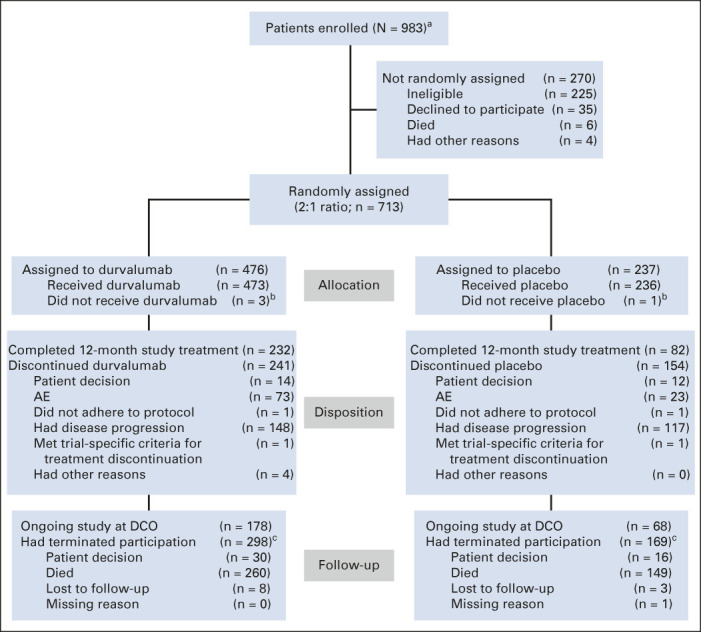
CONSORT diagram. Study data collected up to the DCO date of January 11, 2021. Patients who completed 12 months of study treatment are those for whom the electronic case report form showed that they had received the maximum number of cycles of study treatment. aInformed consent received. bFour patients did not receive their assigned study treatment because of neutropenia (n = 1), worsening chronic obstructive pulmonary disease (n = 1), and patient decision (n = 2). cNine patients (durvalumab, n = 4; placebo, n = 5) who terminated the study because of patient decision have subsequently died; one additional patient (placebo arm) with missing termination reason has subsequently died. AE, adverse event; DCO, data cutoff.
