Abstract
Installing efficient protective immunity by anti‐SARS‐CoV‐2 vaccines is the only current means to overcome coronavirus disease 2019 pandemics. The cellular and humoral immune responses induced with an messenger RNA (mRNA) (BNT162b2) or with a vector (ChAdOx1nCoV‐19) vaccine among Bulgarian healthcare workers (n = 123, aged 23–71 years) were studied in the course of 16 weeks after priming. Receptor‐binding domain (RBD)‐blocking Abs and SARS‐CoV‐2 RBD immunoglobulin A (IgA) were evaluated in parallel with interferon gamma (IFNγ)‐producing virus‐specific T cells. Both vaccines induced RBD‐blocking Abs in 100% of the participants after complete immunization while the levels of protection after a single dose largely varied (22%–98%). Advanced age had a negative impact on the level and longevity of virus‐neutralizing activity induced by one dose mRNA, but not by the vector vaccine. RBD‐binding IgA was detected in 100% of tested donors from the mRNA vaccine cohort, and in 67% of tested from the vector vaccine cohort, at least 1 month after completed immunization. One month after completing mRNA immunization, the number of IFNγ‐producing T cells correlated significantly with the levels of RBD‐specific IgA and virus‐neutralizing activity induced after priming. Enumeration of circulating virus‐specific IFNγ+ T cells is not recommended for evaluation of protective immunity as their detection may require longer stimulation beyond the firstmonth postimmunization.
In conclusion, BNT162B2 and ChAdOx1nCoV‐19 induced potent and comparable humoral and cellular anti‐SARS‐CoV‐2 immune responses, peaking between 10 and 30 days after complete immunization. A single dose of any vaccine did not induce adequate protection in a great part of donors, making the shorter interval between mRNA vaccine doses preferable in the settings of increased risk of infection.
Keywords: COVID‐19 vaccine, immunity, SARS‐CoV‐2 specific T‐cell, virus‐neutralizing antibody
HIGHLIGHTS
One dose BNT162b2 or ChAdOx1nCoV‐19 induced heterogeneous responses, negatively impacted by age in the case of messenger RNA vaccine
RBD‐binding immunoglobulin A is instrumental for monitoring postvaccinal response
Absence of circulating virus‐specific interferon gamma+T does not preclude T‐cell memory for SARS‐CoV‐2
1. INTRODUCTION
Since the end of 2019, the newly emerged SARS‐CoV‐2 has caused over 210 million infections leading to coronavirus disease 2019 (COVID‐19), and over 4.4 million deaths. 1 Unlike its predecessors, SARS‐CoV and MERS‐CoV, SARS‐CoV‐2 has initiated a pandemic with no signs of self‐limitation. 2 Lack of seasonality, resistance outside the host, long incubation period, and possibility of transmission before the appearance of symptoms contribute to its extensive spread.
Adaptive response to SARS‐CoV‐2 relies on two components: virus‐neutralizing antibodies (NAbs) preventing the attachment of free virions to the ACE2 receptor‐expressing host cells, and effector CD8+ and CD4+ T lymphocytes eliminating infected cells by direct or by cytokine‐mediated cytotoxicity. 3 , 4 , 5 In addition, long‐living memory T and B cells are generated, that in case of re‐infection restart production of antibodies and effector T cells, and regulatory T subsets that prevent pathological effects of nonspecific activation. 6
Protective humoral and cellular immunity to SARS‐CoV‐2 has been demonstrated both in animal models and in human. 7 , 8 , 9 Virus‐specific memory T cells were identified 5–17 years after MERS and SARS‐CoV infections. 5 , 6 Recently, SARS‐CoV‐2‐specific bone marrow plasma cells, and persistent germinal center B cell response were reported after recovery from COVID‐19, in favor of long‐lived humoral immune memory. 10 , 11
In the absence of specific antiviral treatment, preventive vaccines aiming to install protective immunity are the only means to limit the spread of infection and the rate of viral mutations. At the end of 2020, a nucleoside‐modified messenger RNA (mRNA)‐based vaccine BNT162B2 (Pfizer/BioNTech) was licensed for application in the EU, followed by the adenovirus vector‐based ChAdOx1 nCoV‐19 (AZD1222) (AstraZeneca). Regardless of the vaccine platform, both aimed at the full‐length viral Spike (S) protein interacting with ACE2 receptors through its receptor‐binding domain (RBD), and mediating viral entry. 12
The immunogenicity of vaccines is of primary scientific and public interest. COVID‐19 vaccines are expected to induce high titers of virus‐specific antibodies, that prevent symptomatic infection, and reduce viral shedding and transmission. In parallel, virus‐specific CD4+ and CD8+ T cells recognizing immunodominant epitopes from S protein, with proper function, are critical for limiting viral replication and spread, and the instauration of memory. 3 , 12
Immunogenicity data published in the course of early clinical studies of both vaccines were limited regarding the assessed parameters and the number of tested participants. 12 Most studies reported data on NAbs and virus‐binding antibodies, 13 , 14 , 15 , 16 , 17 , 18 and only part of them on T‐cell immunity. 14 , 15 , 18 Various methods and modifications of standard protocols were used for evaluation of virus‐specific response, producing hardly comparable relative values. Antibody and T‐cell responses were measured for only a short period after the final immunization. Initial trials involved young or middle‐aged healthy noninfected adults, while minority, professional or risk groups were underrepresented. 12
The application of national vaccination programs brought forward a number of issues, such as the possibility of extending the interval between the two doses of ChAdOx1 nCoV‐19 (AstraZeneca), the immunogenicity of a single vaccine dose, the correlation between humoral and cellular immunity, assessed in parallel, the effects of age, professional risk or comorbidities on the efficacy and duration of immune responses. Real‐life data from particular professional and risk groups, comparing different vaccines and regimens, and assessing in parallel different parameters of humoral and cellular immunity are still lacking.
These are the first data on the immunogenicity of SARS‐CoV‐2 vaccines since the beginning of the national campaign in Bulgaria. Two cohorts of healthcare workers immunized with an mRNA or with a vector vaccine were followed in the course of 16 weeks after the first application. Virus neutralizing activity (VNA) and RBD‐binding immunoglobulin A (IgA) were evaluated alongside virus‐specific interferon gamma (IFNγ)‐secreting T cells, and the impact of age and co‐morbidities was assessed.
2. MATERIALS AND METHODS
This prospective longitudinal study was conducted in two cohorts. Healthcare workers from three hospitals in Sofia (Specialized Infectious Diseases Hospital, Military Medical Academy, Tokuda Hospital) received the first dose of BNT162b2 vaccine (Comirnaty) between 06 and 17 January 2021, and a second dose 21 days later, between 27 January and 17 February 2021, respectively. The vaccine was applied in standard doses (0.3 ml) as specified in the summary of product characteristics (SmPC‐EMA). Serum samples were drawn immediately before the application of the first (D0) and second dose (D21), as well as on D31, D41, D51, and D111. Heparinized peripheral blood for cellular immunity testing was collected on D0, D21, D51, and D111. A total of 71 participants (27 male, 44 female) were enrolled, of whom 61 completed the study until D51, and 49—until D111.
Employees of a vaccine‐producing company (BulBio‐NCIPD Ltd.) received a first dose (0.5 ml) of ChAdOx1 nCoV‐19 (COVID‐19 Vaccine AstraZeneca) between 07 and 22 February 2021, and a second dose on D70, between 26 Apr and 7 May 2021, respectively. The vaccine was applied in standard doses (0.5 ml) as specified in the SmPC‐EMA. Samples were collected immediately before the application of the first (D0) dose, as well as on D30, D60, and D111 after the priming. A total of 49 participants (13 male, 36 female) were enrolled and completed the study.
Volunteers with a positive SARS‐CoV‐2 PCR test in the last 3 months before vaccination or clinical signs of acute infection were excluded from the study. A computer‐based questionnaire on demographic characteristics, comorbidities, allergies, and recent vaccinations was taken by all volunteers eligible for this study before receiving the first dose of the vaccine. The protocol and informed consent were approved by the institutional review board of NCIPD. Written informed consent was obtained from all participants.
Serum samples were tested with SARS‐CoV‐2 RBD IgA assay (Anti‐SARS‐CoV‐2 enzyme‐linked immunoassay [ELISA] IgA, REF#EI 2606‐9601 A, Lot# E210121AL; Euroimmun). Results were evaluated semi‐quantitatively by the ratio of the extinction of the patient sample over the extinction of the calibrator (Es/Ec). According to the manufacturer's instructions, concentrations of IgA corresponding to a ratio greater than 1.1 (Es/Ec) were considered positive, and those below 0.8—negative.
VNA against SARS‐CoV‐2 was evaluated by a blocking ELISA using cPass SARS‐CoV‐2 surrogate virus neutralization test kit (REF#L00847‐C, Lot#A201203; GenScript) after manufacturer's instructions. Briefly, diluted samples were preincubated with horseradish peroxidase‐RBD to allow the binding of circulating RBD‐blocking Abs; the mixture was added to the capture plate precoated with hACE2 receptor protein and revealed with TMB substrate. The absorbance was inversely dependent on the titer of SARS‐CoV‐2 RBD‐blocking antibodies in serum. The inhibition % was calculated as (1−[OD value of the sample/OD value of the negative control]) × 100%. Results above 20% were interpreted as the presence of SARS‐CoV‐2 virus‐neutralizing activity.
Peripheral blood mononuclear cells (PBMC) were isolated by density gradient centrifugation of heparinized blood on Histopaque‐1077 (Sigma‐Aldrich Co. LLC) after a standard protocol. An ELISpot assay was used to enumerate SARS‐CoV‐2‐sensitized T cells producing IFNγ after manufacturer's instructions (T‐Spot.COVID, IVD, lot#VEC3460001, ref#COVID 435.300; Oxford Immunotech). Briefly, PBMC in concentrations adjusted to 2.5 × 105 cells/well were plated in anti IFNγ–coated ELISpot 96‐well plate in the presence of SARS‐CoV‐2 peptides from S protein designed to activate both CD4+ and CD8+ T cells. Negative and positive control of stimulation, medium only and phytohemagglutinin (PHA), respectively, were included in the assay. After overnight culture, cells were washed, and captured IFNγ was revealed using a colorimetric assay. Spots were counted with an automated ELISpot reader (AID). For each stimulation condition, the negative control spot number was subtracted. Results were expressed as spot forming cells (SFC)/106 PBMC. SFC number ≥5 (20/106 PBMC) was considered as the presence of a virus‐specific response, according to manufacturer's instructions (PI‐T‐SPOT.COVID‐IVD‐UK v.3). A limited number of D111 samples were first cultivated on a 96‐well plate for 96 h, in the presence of the same SARS‐CoV‐2 peptides and interleukin (IL)‐2 (102 IU/ml), IL‐2 only, or PHA, and further transferred to an IFNγ–coated ELIspot 96‐well plate to be processed as described above.
Quantitative data were presented as mean (±SD), and qualitative data were presented as percentages. Paired and nonpaired t tests (or the nonparametric Wilcoxon and Mann–Whitney tests when appropriate) were used to compare characteristics between groups, and Spearman's or Pearson correlation coefficient test—to evaluate the association between two or more variables, with GraphPad Prism v. 9.0.0 (GraphPad Software, LLC).
3. RESULTS
3.1. Characteristics of the cohorts
A total of 71 volunteers, 27 male, 44 female, mean age 52 years (range: 23–76), were followed after application of the mRNA vaccine (BNT162b2, Comirnaty). In this cohort 28 donors (39%) had a history of chronic condition: Hashimoto thyroiditis (n = 5), hypertension (n = 11), diabetes (n = 3), tumor (n = 2), ischemic heart disease (n = 2), sarcoidosis (n = 1), Bechterew's disease (n = 1), atopic dermatitis (n = 1), asthma (n = 1), morbus Strumpell (n = 1). Seven reported a recent (up to 3 months before priming) vaccination with influenza vaccine. One volunteer reported a mild COVID‐19 infection, and one another—undefined acute viral infection in October 2020.
A total of 49 volunteers, 13 male, 36 female, mean age 49 years (range: 25–68), were followed after application of the vector vaccine ChAdOx1 nCoV‐19. A total OD 14 (27%) had a history of chronic condition: Hashimoto thyroiditis (n = 3), hypertension (n = 3), diabetes (n = 3), psoriasis (n = 1), chronic renal failure (n = 1), pituitary adenoma (n = 1), liver steatosis (n = 1), chronic pulmonary obstructive disease (n = 1). Four reported allergies caused by drugs, molds, or grass pollen. Three donors had a history of previous mild to moderate SARS‐CoV‐2 infection (June–October 2020). There were no significant differences between the cohorts regarding age, sex distribution, or frequency of chronic conditions.
3.2. Humoral immune response induced by BNT162b2 vaccine
NAbs are considered a better marker of protection, though their titers generally correlate with S‐RBD binding ones. 19 At baseline (D0), 4 donors vaccinated with mRNA vaccine had RBD‐blocking Abs corresponding to VNA >20%. Three weeks after priming with the mRNA vaccine (D21), 72.4% of volunteers developed RBD‐blocking Abs. After completing vaccination on D31, 100% of tested volunteers displayed VNA, and this response was maintained on D41 and D51. Three months after completing vaccination (D111), the share of donors with evidence of VNA significantly dropped (91.8% vs. 100% on D51, p < 0.001) (Figure 1A).
Figure 1.
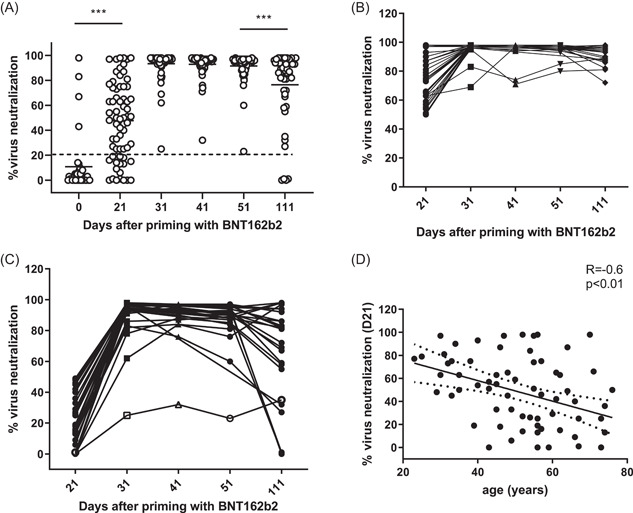
Characteristics of virus‐neutralizing response induced with the mRNA vaccine. (A) Percentage of virus‐neutralizing activity (VNA) calculated as described in Section 2 using the absorbance of the anti‐SARS‐CoV‐2 RBD‐blocking Abs. Dotted line corresponds to the cut‐off level (20%) ***p < 0.001. (B, C) Individual dynamics of responses in donors who had >50% and <50% VNA, respectively, on D21 to D111. A 59‐year‐old female donor with Hashimoto thyroiditis and low VNA at all‐time points is depicted with open symbols. (D) Correlation between the age of immunized and VNA on D21 after priming with the mRNA vaccine. mRNA, messenger RNA
As the levels of VNA on D21 widely varied: mean (min–max) 48 (23–98) %, we asked whether D21 low responses reflected immune deficiency or were corrected after the second dose. Therefore, we analyzed separately the dynamics of high (>50% VNA) and low (<50% VNA) D21 responses (Figure 1B,C). In the first subgroup, 100% of the volunteers developed RBD‐blocking Abs, with a high level of VNA detected on D31, D41, D51, and D111: (mean) 96%, 95%, 95%, 91%, respectively. Overall, 34 volunteers (48%) from the cohort, had a low D21 response, and 18 (25%) did not develop RBD‐blocking Abs after priming (VNA <20%), both represented by the second subgroup. After the second dose, on D31, D41, and D51 VNA was detected in all 34 low responders, though at a lower level as compared to the other subgroup: (mean) 91%, 91%, and 88%, respectively. On D111 12% of the low responders had lost their VNA (mean 66% vs. 91% in the other subgroup, p < 0.05). One single donor from the low responders' subgroup displayed significantly lower VNA levels at all time points. It was a 59‐year‐old female with Hashimoto thyroiditis. No one in this subgroup had detectable VNA on D0 versus 4 out of 38 donors (10%) in the first one (Figure 1A). However, the difference between D0 VNA values was not significant enough (p = 0.08) to explain the high variability of induced responses.
VNA response to one dose mRNA vaccine (D21) correlated significantly and negatively with the age of the immunized (R = −0.6, p < 0.01) (Figure 1D), but was not associated with the presence of a chronic condition. In contrast, no such negative correlation was seen on D111 (data not shown). Thus, a low/absent VNA after the first dose was not necessarily associated with immune deficiency and did not preclude VNA response after a complete immunization. Although advanced age requires completion of immunization schedule on short notice, it does not impact the level or half‐life of virus‐neutralizing activity in peripheral blood.
Immediate protection depends strongly on the availability of SARS‐CoV‐2 specific IgA. 20 Therefore, RBD‐specific IgA responses were evaluated in addition. As expected, one dose mRNA vaccine‐induced IgA levels (mean ± SD 4.07 ± 3.1 on D21 vs. 2.1 ± 3.2 at baseline, p < 0.0001), that were further increased after complete immunization (6.05 ± 3.1 on D51, p < 0.001 as compared to D21), (Figure 2A). Intriguingly, a subgroup of donors had significantly higher levels of RBD‐specific IgA (above the mean ± SD) as compared to the rest of the group on D21 (10.8 ± 1.2 vs. 2.6 ± 1.7, p < 0.0001), and D51 (9.5 vs. 5.4, p < 0.01), (Figure 2B). Nine donors (13% of tested) had no IgA response after the first dose (D21) but all of them responded 30 days after completed vaccination (D51), represented together with the rest of the group in Figure 2C. No correlations were established between IgA and total RBD‐blocking Abs on D51, nor with the age of donors (data not shown). Noteworthily, the subgroup of donors with higher levels of RBD‐specific IgA had higher baseline values of VNA: VNA above 20% in 6/11 donors (56%), mean VNA 3.7% versus 0.9% (p < 0.01). Increased RBD‐IgA was associated with higher levels of VNA (9.5 ± 3.0 vs. 5.4 ± 2.8) but not with the age of donors (data not shown). Since only two donors had a history of prior viral infection, the presence of RBD‐blocking Abs could be explained by asymptomatic recurrent contacts with the virus.
Figure 2.
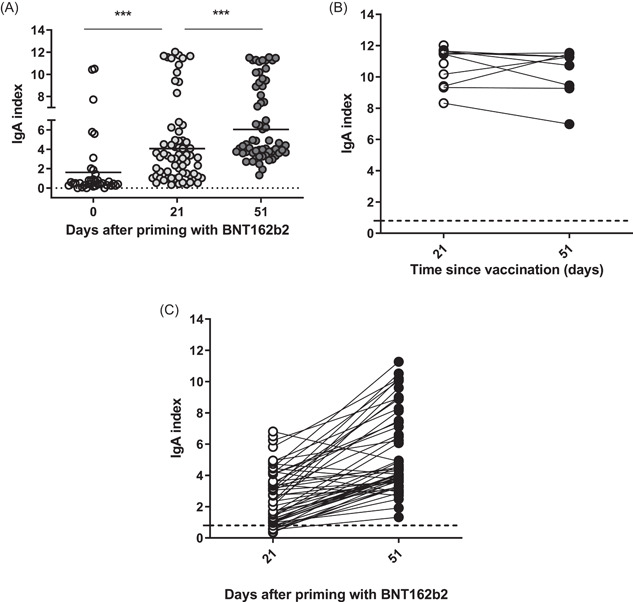
Characteristics of virus‐specific IgA response induced with the mRNA vaccine. (A) Individual values of RBD‐binding IgA index on D0, D21, and D51. Means are denoted. Dotted line corresponds to the cut‐off level (0.8), ***p < 0.001. (B) Dynamics of individual RBD‐specific IgA responses in the subgroup with high IgA values on D21 to D51. (C) Dynamics of individual RBD‐specific IgA responses in the rest of the volunteers. IgA, immunoglobulin A; mRNA, messenger RNA; RBD, receptor‐binding domain
3.3. Humoral immune response induced by ChAdOx1 nCoV‐19 vaccine
The vector vaccine ChAdOx1 nCoV‐19 (Covid‐19 Vaccine AstraZeneca) was applied in two doses at 10‐week intervals. Volunteers were tested twice before the second application, on D30 and D60, and 6 weeks after the boost (D111) to have comparable time‐points with the first cohort. On D0, two of the donors with prior SARS‐CoV‐2 infection had already VNA (67% and 93%, respectively, Figure 3A). On D30, 92% had developed SARS‐CoV‐2 specific VNA. One month later (D60) this share dropped to 76%, while 6 weeks after the second application (D111) it rose to 100%. The mean levels of detectable VNA on D30, D60, and D111 were 57.5; 39.3 and 84.8, respectively (Figure 3A). In fact, while not reaching statistical significance, the levels of RBD‐blocking Abs induced after priming with the vector vaccine on D30 were superior to those induced with one dose mRNA vaccine on D21 (mean ± SD 57.5 ± 27 vs. 47.7 ± 31, p = 0.075). However, these levels decreased to 39.3% versus 57.5% on D60 (p < 0.01).
Figure 3.
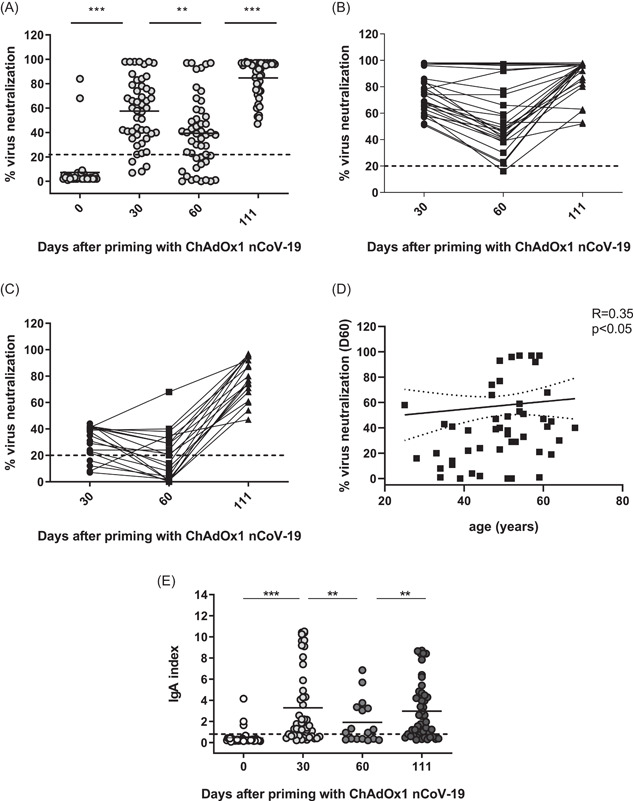
Characteristics of virus‐neutralizing activity (VNA) and virus‐specific IgA responses induced after immunization with vector vaccine. (A) Summarized data for VNA on D0, D30, D50, and D111 calculated as described in the Section 2 using the absorbance of the anti‐SARS‐CoV‐2 RBD‐blocking Abs. Dotted line corresponds to the cut‐off level (20%), **p < 0.01, ***p < 0.001. (B, C) Individual dynamics of responses in donors who had >50% and <50% VNA, respectively, on D30 to D111. (D) Correlation between the age of immunized and VNA on D60 after priming with vector vaccine. (E) Individual values of RBD‐binding IgA index on D0, D30, D60, and D111. Means are denoted. Dotted line corresponds to the cut‐off level (0.8). IgA, immunoglobulin A
The separate analysis of the dynamics of D30 responses showed that all high (>50% VNA) D30 responders had a lower or comparable VNA on D60 (mean 57% vs. 76% on D30, p < 0.001), and all increased their SARS‐CoV‐2‐specific VNA on D111 as compared to D30 (89% vs. 76%, p < 0.001), (Figure 3B). Overall, 80% from the low (<50% VNA) D30 responders displayed some VNA (mean 30%), and only 50% had VNA on D60 (mean 19.9%, p < 0.001 as compared to D30). However, 100% had a significantly increased VNA 1 month after the second injection (78% vs. 19.9%, p < 0.001) (Figure 3C). Unlike the cohort immunized with mRNA vaccine, no correlation existed between the age of donors and VNA responses on D30 (data not shown). A weak direct correlation (R = 0.35, p < 0.05) was established between the age and VNA levels on D60 showing that elderly individuals responded somewhat better to one‐dose immunization with vector vaccine (Figure 3D).
The priming with vector vaccine induced an RBD‐IgA response in 68% of donors on D30 (mean 3.3 ± 3.5 vs. 0.5 ± 0.7 on D0, p < 0.001), this response waned on D60 when 48% were positive (mean 2.0 ± 2.1), and was boosted again after complete immunization: 69% positive on D111 (mean 3.0 ± 2.7), (Figure 3E). While RBD‐IgA induced by one dose of mRNA (D21) or vector vaccine (D30) were not significantly different (4.07 ± 3.1 vs. 3.3 ± 3.5, p > 0.05), 30 days after completed immunization (on D51) the mRNA cohort had a higher level of RBD‐IgA as compared to the vector vaccine cohort (D111, 6.05 ± 3.1 vs. 3.3 ± 3.5, p > 0.001). Noteworthy, the mRNA cohort was also characterized with a significantly higher baseline RBD‐IgA level (2.1 ± 3.2 vs. 0.5 ± 0.7), probably due to the higher exposure of volunteers in the mRNA cohort who work in hospitals.
3.4. SARS‐CoV‐2 specific cellular immune response induced after the vaccination
The efficiency and longevity of virus‐specific antibody response depend on the induction of cellular immunity. 3 Most clinical studies reported data about the early instauration of cellular immunity (D14 and D28). We evaluated the number of IFNγ‐secreting lymphocytes (or SFC) in response to stimulation with S1 peptides, at later time points. At baseline (D0) no positive responses were detected in either cohort. On D51 and D111 after priming with mRNA vaccine, the mean number of SFC was 115 and 57, respectively, p < 0.05) (Figure 4A). On D51, 4 out of 49 tested donors (6%) did not display virus‐specific IFNγ secretion (<20 SFC/106 PBMC after subtraction of the negative control). However, all of them were positive on D111, indicating the presence of virus‐specific T‐cell memory (data not shown). On the other hand, 8 donors had less than 20 SFC/106 PBMC on D111, after a positive response on D51.
Figure 4.
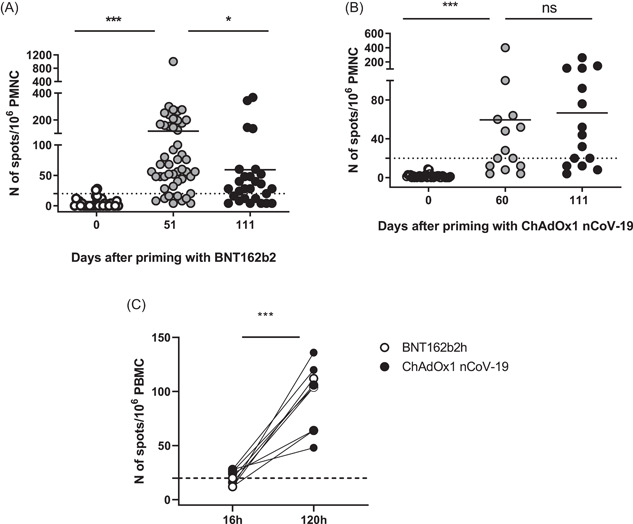
Postvaccinal SARS‐CoV‐2 specific cellular immune response. (A) Individual numbers of SFC on D0, D51, and D111 after immunization with mRNA vaccine, *p < 0.05, ***p < 0.001. (B) Individual numbers of SFC on D0, D60, and D111 after immunization with the vector vaccine, ns p > 0.05. (C) Comparison between the number of SFC after short‐term (16 h) and long‐term (120 h) stimulation with SARS‐CoV‐2 S peptide pool. Dotted lines correspond to the cut‐off level of the test (20 SFC/106 PBMC). mRNA, messenger RNA; PBMC, peripheral blood mononuclear cells
The mean SFC number after priming with vector vaccine was 59 on D60, and was not significantly different on D111, 6 weeks after completed immunization (67 vs. 59, p > 0.05) (Figure 4B). This value was also comparable to the T cell response elicited 12 weeks after completed immunization with the mRNA vaccine, on D111 (67 vs. 57, p > 0.05). With the vector vaccine, 7 donors did not display virus‐specific IFNγ secretion on D60, and two of them did not display virus‐specific T cells on D111, either. A total of six donors were negative on D111 (Figure 4B).
Importantly, all donors with low/absent cellular activity on D111 had in parallel significant levels of VNA. We reasoned that virus‐specific effector T cells might not be readily detectable in peripheral blood at later time points, unlike the effector‐memory or memory T cells. As the latter need a longer time for IFNγ expression, we applied a modified protocol for in vitro stimulation (see Section 2). Briefly, preserved PBMC from donors with a negative T‐cell response on D111 after priming with either mRNA or vector vaccine were stimulated in the presence or in the absence of SARS‐CoV‐2 S peptide pool, and IL‐2 (102 IU/ml) for 96 h. On D4 the cells were collected and restimulated on an ELISpot plate for one additional night before the read‐out. After the long‐term stimulation, all samples that tested negative on D111 gave a positive signal (Figure 4C).
The number of SFC induced with either mRNA or vector vaccine on D51 and D60 respectively correlated significantly with the levels of VNA on D21‐30 (r = 0.4, p < 0.01) and D51‐60 (r = 0.4, p < 0.01), RBD‐specific IgA (for mRNA) (r = 0.34, p < 0.05) detected on D21 and D51 (Figure 5A–C), which was not valid for SFC detected on D111(data not shown). The inverse correlation with age did not reach statistical significance (p = 0.09, data not shown).
Figure 5.
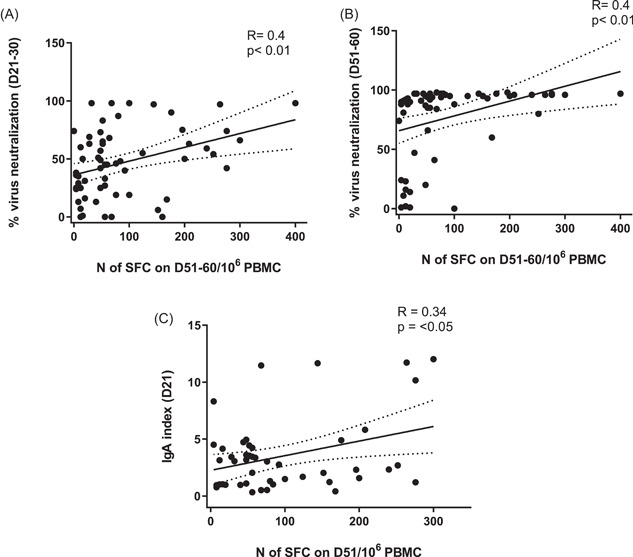
Correlations between cellular immune responses, elicited with mRNA or vector vaccine expressed by the individual numbers of SFC, and: (A) virus‐neutralizing activity on D21‐30, (B) virus‐neutralizing activity on D51‐60, (C) RBD‐binding IgA on D21 for mRNA cohort. IgA, immunoglobulin A; mRNA, messenger RNA; RBD, receptor‐binding domain
4. DISCUSSION
To date, SARS‐CoV‐2 vaccines and mass immunization remain the only working strategy for limiting virus spread and decreasing the speed of mutations. The characteristics of postvaccinal immune response not only determine the immediate protection of the immunized and their close contacts but have a long‐term impact on the epidemic process. Both virus‐specific antibodies and cytotoxic T cells are necessary to limit infection, and long‐living memory cells warrant future protection.
According to the National COVID‐19 Vaccination Plan, the first immunization phase in Bulgaria included healthcare workers, to preserve medical services of prime importance. We compared antibody and cellular immune responses after immunization with an mRNA (BNT162b2, Comirnaty) and with a vector vaccine (ChAdOx1nCoV‐19, COVID‐19 Vaccine AstraZeneca). The generation of RBD‐blocking Abs, RBD‐specific IgA, and IFNγ+ virus‐specific T cells were evaluated in parallel, at similar time points after the priming (D21‐30, D51‐60, and D111). According to the application schedules, the mRNA cohort was followed for 12 weeks, and the vector vaccine cohort—for 6 weeks after completed vaccination. The follow‐up period of 111 days was extended in comparison to other studies. Early clinical studies on immunogenicity vary from 35 to 43 days for mRNA 16 , 17 , 18 to 56 days postpriming for AstraZeneca vaccine. 14 , 15 Real‐life studies evaluated immunogenicity at 35 21 , 22 and 40–42 days 23 , 24 after priming. A single pilot study reported data at 31 days after the second dose of the Pfizer‐BioNTech mRNA vaccine. 25 Partial results from an ongoing phase1 trial demonstrated mRNA1273‐elicited binding and NAbs in 33 healthy adult participants at 180 days after completed immunization, 26 and a limited study on 20 participants demonstrated durable humoral and cellular immune responses with minimal decreases at least 8 months after immunization with Ad26.COV2.S vaccine. 27
Postvaccinal humoral responses are evaluated either by quantifying RBD‐/S‐binding antibodies or antibodies that block infection. Neutralization assays are clearly more informative but also technically demanding. Therefore, we applied an immunoassay detecting the antibodies that block the binding of the S protein to a soluble form of the ACE2 receptor. Early clinical trials data comparing the immunogenicity of BNT162b2, and BNT162b1 based on a limited number of tested donors, reported the highest VNA on D28 and D35 after priming (i.e., 7 and 14 days after the second dose). 17 The most important postlicensing data come from Israel where the COVID‐19 vaccination program was completed exclusively with BNT162b2 vaccine. 21 , 22 A cohort study of Israeli healthcare workers, demonstrated a robust antibody response on D14 after the first dose, peaking on D30, approximately 10 days after the second one. 21 Another prospective cohort study in healthcare workers identified NAb responses at Week 3 (D21) postpriming in 71% of tested, which rapidly increased on D31 in 96.5% of the tested. 22 Early data on the immunogenicity of ChAdOx1 nCoV‐19 reported VNA in 91%–100% of tested on D28 after a single dose, and in 100% of tested on D35, D42, and D56 after a prime‐boost applied 4 weeks appart. 14
It is difficult to conclude on the superiority of induced immune responses when comparing separate studies based on different methods and relative units. Few have compared different types of vaccines in similar populations. 12 , 23 , 25 According to our results, both vaccines induced comparable VNA peaking 10–30 days after the second application (D31–D51 for the mRNA and D111 for the vector vaccine), detectable in 100% of tested volunteers.
The decline of VNA in peripheral blood is another important parameter of vaccine efficacy. Studies on BNT162b2 reported insignificant decline but data was limited to D28–D35 21 or D60 after priming. 22 We followed VNA response to the mRNA vaccine for a considerably longer time (D111 after priming or 12 weeks after complete immunization), and report a significantly decreased level of VNA: 76.5% on D111 versus 91.5% on D51 (p < 0.001), with 9% of tested donors displaying no VNA at all.
According to a phase 1/2 trial data, 2 weeks after application of two doses of ChAdOx1 nCoV‐19 (AZD1222), 8 weeks apart (on D70), VNA was significantly increased as compared to VNA before the boost. 13 Pooled data from three single‐blind randomized trials, exploring the effect of extended prime‐boost interval on the immunogenicity of ChAdOx1 nCoV‐19, demonstrated higher IgG anti‐S and NAb titers with an interval of over 12 weeks as compared to an interval of 6 weeks in donors aged 18–55 but not donors aged above 55. 28
In our hands, two doses delivered 10 weeks apart significantly increased VNA documented 6 weeks later, on D111 (84.7 vs. 39.3% on D60, p < 0.001). This difference was valid for the entire tested group and was not associated with the age of donors.
Importantly, a single dose of either vaccine did not deliver sufficient protection in terms of virus‐neutralizing activity in all studied donors. The responses were heterogeneous, with 73% of tested responding to the mRNA vaccine on D21 and 80%—to the vector vaccine on D30. These rates were comparable to the results reported by Lustig et al. 22 for BNT162b2 (71% on D21) but lower than the 91%–100% on D28 reported for ChAdOx1 nCoV‐19 (AZD1222) by Folegatti et al. 14
In our hands, although superior to those induced with the mRNA vaccine on D21 versus D30 for vector vaccine, the levels of RBD‐blocking Abs decreased by 32% on D60, similarly to a reported waning of IgG anti‐S antibody levels by 34% from D28 to D90 after a single dose of ChAdOx1 nCoV‐19. 28 Therefore, a two‐dose regimen should be followed strictly, and prolonging the interval between doses should not be encouraged.
A significant inverse correlation was observed between VNA levels and age on D21 after priming with mRNA vaccine. Importantly, after the booster, age was no longer a factor for the strength or the waning of antibody response. Other studies also reported an association between lower antibody concentrations induced with either mRNA or vector vaccine and older age (≥65 or 70 years), disappearing on D42 postvaccination. 21 , 22 , 25 In addition, lower antibody titers were associated with male sex, immunosuppression, and specific comorbidities: diabetes, hypertension, heart disease, and autoimmune diseases. 21 , 22 We did not observe such associations in our study, probably because of the small number of reported comorbidities, and uneven sex distribution within the groups. Importantly, we did not observe an age‐related effect on VNA levels induced with one dose of the vector vaccine. In contrast, a weak positive correlation on D60 revealed a slower waning of virus‐neutralizing response in the elderly.
Few postvaccinal studies have explored virus‐specific IgA responses.. Lustig et al., reported RBD‐binding IgA response in only 3% of participants on D7 after priming with BNT162b2, with substantial increase to 43% on D14, and a maximum of 85% 7 days after boost (D28). 22 Ewer et al. 15 reported SARS‐CoV‐2 spike‐specific IgA peaking on D28 after priming with ChAdOx1 nCoV‐19. In our study, a higher proportion of donors (87%) responded to the first dose of mRNA vaccine, and 4 weeks after complete vaccination (D51) 100% of the tested were positive. In addition, a subgroup of donors with significantly higher levels of RBD‐specific IgA, including the baseline, was delineated. This was in contrast with the vector vaccine which elicited IgA response in 69%, and 67% of tested donors on D30, and D111, respectively.
Age, chronic conditions like diabetes, and previous infections may impact virus‐specific IgA response. No significant differences regarding age and co‐morbidities existed between our two cohorts. Only two cases of previous COVID‐19 infection were reported among donors with an initially high level of virus‐specific IgA in the mRNA cohort. Stably elevated IgA levels after mRNA immunization might reflect frequent contact with the virus with sub‐ or no clinical manifestations which is natural for healthcare workers, and confirm the concept of a single dose vaccine after natural immunization. 29
Virus‐specific T cell response is a key component of anti‐SARS‐CoV‐2 immunity. About 10% of immunized may not develop IgG virus‐specific responses, and viral mutations could limit the efficacy of NAbs. Therefore, a vigorous cellular response would be critical to restrict infection. Clinical studies on postvaccinal T‐cell responses are limited in number, tested participants, and duration of follow‐up. Sahin et al. 18 reported virus‐specific CD4+ and a CD8+ T cell responses in 94% and 80% of tested 7 days after completing BNT162b1 vaccination (D28), with IFNγ being the major secreted cytokine. Similarly, the other approved mRNA vaccine (mRNA‐1273) induced CD4+ Th1‐biased responses, and detectable CD8+ T cell responses. 3 Clinical trials data on ChAdOx1 nCoV‐19 reported initial cellular responses on D7, that developed in all participants by D14, and were still detectable on D56. 14 Alternative vector‐based vaccines elicited similar IFNγ+ T cell responses peaking on D14, and detectable on D28. 30
Based on published data, we assessed T cell activity using IFNγ‐based ELISpot assay on D51 (expecting a fully developed response), and on D111 to explore its longevity. Viral‐specific effector T‐cells were not readily detectable in peripheral blood 1 month after completed immunization with mRNA vaccine (D51) in 8% of donors and further waned until D111 in 20% of donors. Likewise, 27% of those immunized with vector vaccine had no detectable IFNγ+ T cells on D111. However, virus‐specific T cell activity was documented in all tested nonresponders after additional stimulation. These results confirm the induction of virus‐specific memory T cells that need a longer period of stimulation to express IFNγ and corroborate with the presence of high levels of RBD‐blocking Abs and RBD‐binding IgA. Therefore, routine evaluation of virus‐specific T cell response in peripheral blood beyond 1 month after immunization is of no practical value. In case of an absent or inadequate humoral immune response, a modified activation protocol for detection of virus‐specific T cells should be considered.
In conclusion, BNT162B2 and ChAdOx1nCoV‐19 induced potent and comparable humoral and cellular anti‐SARS‐CoV‐2 immune responses in Bulgarian healthcare workers, peaking between 10 and 30 days after complete immunization. One dose of any vaccine did not induce a substantial immune response correlating to protection for a great part of advanced age donors. Therefore, due to the shorter interval between doses, mRNA vaccines should be considered in settings of increased risk of infection combined with advanced age. RBD‐binding IgA is a practical correlate of protection for at least 1 month after complete immunization. Routine evaluation of virus‐specific IFNγ response in peripheral blood is not recommended, as it is well correlated with VNA. Also, detection of virus‐specific T cells may require longer stimulation beyond the first postimmunization month.
CONFLICT OF INTERESTS
The authors declare that there are no conflict of interests.
AUTHOR CONTRIBUTIONS
Maria Nikolova: designed the study, analyzed data, and wrote the paper. Yana Todorova: performed the evaluation of virus‐specific IgA and T cells, and analyzed data. Radoslava Emilova: performed the evaluation of virus‐specific IgA, and analyzed data. Iva Trifonova: performed the evaluation of RBD‐blocking Abs, TD performed the evaluation of RD‐blocking Abs. Nina Petrova‐Yancheva: enrolled volunteers, and collected data. Tatyana Chervenyakova: enrolled volunteers. Elena Dragusheva: enrolled volunteers, and collected data. Georgi Popov: enrolled volunteers. Iva Christova: designed the study, and wrote the paper.
PEER REVIEW
The peer review history for this article is available at https://publons.com/publon/10.1002/jmv.27572
ACKNOWLEDGEMENTS
Supported by ERDF through OP Science and Education for Smart Growth 2014‐2020 (Grant: BG05M2OP001‐1.002‐0001‐C04).
Nikolova M, Todorova Y, Emilova R, et al. Induction of humoral and cellular immune responses to COVID‐19 mRNA and vector vaccines: A prospective cohort study in Bulgarian healthcare workers. J Med Virol. 2022;94:2008‐2018. 10.1002/jmv.27572
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available from the corresponding author upon reasonable request. Requests for access to the study data can be submitted to the corresponding author.
REFERENCES
- 1. Coronavirus WHO (COVID‐19) Dashboard. https://covid19.who.int
- 2. Rabaan AA, Al‐Ahmed SH, Haque S, et al. SARS‐CoV‐2, SARS‐CoV, and MERS‐COV: a comparative overview. Infez Med. 2020;28(2):174‐184. [PubMed] [Google Scholar]
- 3. DiPiazza AT, Graham BS, Ruckwardt TJ. T cell immunity to SARS‐CoV‐2 following natural infection and vaccination. Biochem Biophys Res Com. 2021;538:211‐217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Wagner GDS. Impact of virus genetic variability and host immunity for the success of COVID‐19 vaccines. Biomed Pharmacother. 2021;136(4):111272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Lombardi A, Bozzi1 G, Ungaro R. Immunological consequences of immunization with COVID‐19 mRNA vaccines: preliminary results. Front Immunol. 2021;12:657711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Nikolova M, Lelievre JD, Carriere M, Bensussan A, Levy Y. Regulatory T cells differentially modulate the maturation and apoptosis of human CD8+ T‐cell subsets. Blood. 2009;113(19):4556‐4565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. McMahan K, Yu J, Mercado NB, et al. Correlates of protection against SARS‐CoV‐2 in rhesus macaques. Nature. 2020;590:630‐634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Huang AT, Garcia‐Carreras B, Hitchings MDT, et al. A systematic review of antibody mediated immunity to coronaviruses: kinetics, correlates of protection, and association with severity. Nat Commun. 2020;11:1‐16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Le Bert N, Tan AT, Kunasegaran K, et al. SARS‐CoV‐2‐specific T cell immunity in cases of COVID‐19 and SARS, and uninfected controls. Nature. 2020;584:457‐462. [DOI] [PubMed] [Google Scholar]
- 10. Turner JS, Kim W, Kalaidina E, et al. SARS‐CoV‐2 infection induces long‐lived bone marrow plasma cells in humans. Nature. 2021;595:421‐425. [DOI] [PubMed] [Google Scholar]
- 11. Turner JS, O'Halloran JA, Kalaidina E, et al. SARS‐CoV‐2 mRNA vaccines induce persistent human germinal centre responses. Nature. 2021;596:109‐113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Klasse PJ, Nixon DF, Moore JP. Immunogenicity of clinically relevant SARS‐CoV‐2 vaccines in nonhuman primates and humans. Sci Adv. 2022;7(12):eabe8065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Barrett JR, Belij‐Rammerstorfer S, Dold C, et al. Phase 1/2 trial of SARS‐CoV‐2 vaccine ChAdOx1 nCoV‐19 with a booster dose induces multifunctional antibody responses. Nature Med. 2021;27(2):279‐288. [DOI] [PubMed] [Google Scholar]
- 14. Folegatti PM, Ewer KJ, Aley PK, et al. Safety and immunogenicity of the ChAdOx1 nCoV‐19 vaccine against SARS‐CoV‐2: a preliminary report of a phase 1/2, single‐blind, randomised controlled trial. Lancet. 2020;396:467‐478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Ewer KJ, Barrett JR, Belij‐Rammerstorfer S, et al. T cell and antibody responses induced by a single dose of ChAdOx1 nCoV‐19 (AZD1222) vaccine in a phase 1/2 clinical trial. Nature Med. 2021;27(2):270‐278. [DOI] [PubMed] [Google Scholar]
- 16. Walsh EE, Frenck RW Jr, Jr. , Falsey AR, et al. Safety and immunogenicity of two RNA‐based Covid‐19 vaccine candidates. N Engl J Med. 2020;383:2439‐2450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Mulligan MJ, Lyke KE, Kitchin N, et al. Phase I/II study of COVID‐19 RNA vaccine BNT162b1 in adults. Nature. 2020;586:589‐593. [DOI] [PubMed] [Google Scholar]
- 18. Sahin U, Muik A, Derhovanessian E Concurrent human antibody and TH1 type T‐cell responses 2 elicited by a COVID‐19 RNA vaccine https://www.medrxiv.org/content/ 10.1101/2020.07.17.20140533 [DOI]
- 19. Gaebler C, Wang Z, Lorenzi JCC. Evolution of antibody immunity to SARS‐CoV‐2. Nature. 2021;591(7851):639‐644M. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Sterlin D, Mathian A, Miyara M, et al. IgA dominates the early neutralizing antibody response to SARS‐CoV‐2. Sci Transl Med. 2021;13:eabd2223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Grupel D, Gazit S, Schreiber L, et al. Kinetics of SARS‐CoV‐2 anti‐s IgG after BNT162b2 vaccination. Vaccine. 2021;39(38):5337‐5340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Folegatti PM, Ewer KJ, Aley PK, et al. BNT162b2 COVID‐19 vaccine and correlates of humoral immune responses and dynamics: a prospective, single‐centre, longitudinal cohort study in health‐care workers. Lancet. 2020;396:467‐478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Bertrand D, Hamzaoui M, Lemee V, et al. Antibody and T cell response to SARS‐CoV‐2 messenger RNA BNT162b2 vaccine in kidney transplant recipients and hemodialysis patients. J Am Soc Nephrol. 2021;32(9):2147‐2152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Tut G, Lancaster T, Krutikov M, et al. Profile of humoral and cellular immune responses to single doses of BNT162b2 or ChAdOx1 nCoV‐19 vaccines in residents and staff within residential care homes (VIVALDI): an observational study. The Lancet/Healthy Longevity. 2021;2:544. 10.1016/S2666-7568(21)00168-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Lange A, Borowik A, Bochenska J, Bochenska J, Rossowska J, Jaskuła E. Immune response to COVID‐19 mRNA vaccine—a pilot study. Vaccines. 2021;9:488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Doria‐Rose N, Suthar MS, Makowski M, et al. Persistence through 6 months after the second dose of mRNA‐1273 Vaccine for Covid‐19. N Engl J Med. 2021;384(23):2259‐2261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Barouch D, Stephenson KE, Sadoff J. Durable humoral and cellular immune responses 8 months after Ad26.COV2.S vaccination. N Engl J Med. 2021;385(10):951‐953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Voysey M, Costa Clemens SA, Madhi SA, et al. Single‐dose administration and the influence of the timing of the booster dose on immunogenicity and efficacy of ChAdOx1 nCoV‐19 (AZD1222) vaccine: a pooled analysis of four randomized trials. Lancet. 2021;397:881‐891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Havervalla S, Markinga U, Greilert‐Norina N. Antibody responses after a single dose of ChAdOx1 nCoV‐19 vaccine in healthcare workers previously infected with SARS‐CoV‐2. EBioMedicine. 2021;70:103523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Havervall S, Marking U, Greilert‐Norin N, et al. Safety, tolerability, and immunogenicity of a recombinant adenovirus type‐5 vectored COVID‐19 vaccine: a dose‐escalation, open‐label, non‐randomised, first‐in‐human trial. Lancet. 2020;395:1845‐1854. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request. Requests for access to the study data can be submitted to the corresponding author.


