Abstract
To analyze the clinical presentation and outcomes of myocarditis after administration of the severe acute respiratory syndrome coronavirus 2 (SARS‐CoV‐2) messenger RNA (mRNA) vaccine. Nine case series and 15 case reports (74 patients) of myocarditis after administration of the BNT162b2 or mRNA‐1273 vaccine were reviewed from PubMed, Scopus, Embase, and Web of Science. We analyzed clinical manifestations, diagnostic findings, and outcomes. In addition, we performed a pooled analysis and investigated risk factors leading to admission to the intensive care unit and recovery with conservative care. Most patients were male (94.6%), and the median age (range) was 17.6 (14–70) years. Patients who received the BNT162b2 (n = 58, 78.4%) vaccine presented fewer systemic symptoms and left ventricular dysfunction than mRNA‐1273 recipients. Although patients under 20 years experienced more fever and myalgia, they had better ejection fraction and less prominent myocardial inflammation in magnetic resonance imaging than older patients. The clinical course of all patients was favorable without mortality, and one‐third of patients resolved with conservative care alone. Risk factor analyses revealed that patients with gastrointestinal symptoms required intensive care (odds ratio: 20.3, 95% confidence interval 1.90–217, p = 0.013). The risk of fatality in myocarditis subjected to mRNA vaccination seems to be low. However, patients with gastrointestinal symptoms received more intensive care, and a significant proportion of patients recovered with conservative management.
Keywords: BNT162b2, COVID‐19 vaccine, mRNA‐1273, myocarditis, vaccine‐induced myocarditis
1. INTRODUCTION
Coronavirus disease (COVID‐19), caused by severe acute respiratory syndrome coronavirus‐2 (SARS‐CoV‐2), has resulted in a global health and economic crisis, causing a total of 228 541 752 confirmed cases and 4 691 866 deaths, as of September 20, 2021. Globally, vaccination against the virus, to achieve herd immunity, has become the most pressing issue for mitigating the global threat of the virus. 1 Currently, four vaccines have been approved either by the European Medicines Agency (EMA) or by the United States Food and Drug Administration (FDA), including two messenger RNA (mRNA)‐based vaccines—BNT162b2 (Pfizer‐BioNTech) and mRNA‐1273 (Moderna)—and two recombinant adenoviral vector vaccines—ChAdOx1 nCoV‐19 (Astra‐Zeneca), and Ad26.COV2.S (Johnson & Johnson/Janssen). These vaccines have been developed and distributed at an unprecedented pace, and they are highly effective in protecting against SARS‐CoV‐2 infection by neutralizing antibodies. 2 , 3 , 4 They have been proven safe in large‐scale trials in which adverse cardiovascular effects related to the vaccine were studied, wherein an incidence of <0.05% was reported, and myocarditis was not reported. 3 However, there have been emerging concerns regarding myocarditis as rare complication of mRNA‐based COVID‐19 vaccines, especially in young adults and adolescent males. 5
According to the Advisory Committee on Immunization Practices from the Centers for Disease Control and Prevention (CDC), after over 365 million doses administered by August 26, 2021, there were 1903 reports of possible myopericarditis cases in the Vaccine Adverse Event Reporting System, 6 of which 1839 (96.6%) were following the mRNA vaccine. Additional analyses by the CDC Vaccine Safety Datalink, with weekly monitoring, performed using prespecified outcomes of interest, revealed an increased risk of myocarditis after the administration of COVID‐19 mRNA vaccines, as compared with unvaccinated individuals or those who received non‐mRNA vaccines during the same calendar days (rate ratio, 15.6 [95% confidence interval [CI], 6.1–47.2]; for individuals aged 12–39 years, during the 7‐day risk interval after vaccination, adjusted for site, age, sex, race/ethnicity, and calendar date). The estimated rate of myocarditis was 12.6 cases per million doses among individuals aged 12–39 years, receiving the second dose of the COVID‐19 mRNA vaccine. The Israeli Ministry of Health also reported 148 cases of myocarditis among 10.4 million vaccinated individuals, occurring within 30 days of receiving the mRNA vaccination. Among over 528 million doses of vaccines administered to people in the European Union and the European Economic Area, as of the beginning of September 2021, 392 million doses of BNT162b2 and 54.2 million doses of mRNA‐1273 were administered. Of which, 2360 cases of myocarditis were reported among individuals who received BNT162b2, and 1050 cases were reported among those who received mRNA‐1273. The estimated incidence of myocarditis after receiving the COVID‐19 mRNA vaccine was 7.7 cases per million. Additionally, there were 54 deaths reported, suggesting that it is a very important issue that needs to be addressed and should not be overlooked. 7 , 8
Thus far, case reports and case series of myocarditis related to mRNA vaccine are accumulating; however, owing to insufficient sample size, it is difficult to draw consistent, significant conclusions regarding their clinical presentation and treatment. Moreover, no study has analyzed the differential outcomes of COVID‐19 vaccine‐associated myocarditis, such as recovery with conservative care and intensive care unit (ICU) admission, along with associated risk factors. Given this background, the present systematic review aimed to study previously published case reports and case series associated with COVID‐19 mRNA vaccine‐related myocarditis, and investigate the risk factors related to clinical outcomes. Although our findings are limited in their generalizability, our study can provide clinicians a comprehensive understanding of this rare, adverse event, and also support people who require more information before getting vaccinated.
2. METHODS
2.1. Search strategy and selection criteria
This systematic review was performed in agreement with the Preferred Reporting Items for Systematic Reviews and Meta‐Analyses Protocols (PRISMA‐P, Table S1). 9 As reports are being updated every day, a rapid review was conducted to summarize all published cases of myocarditis related to mRNA vaccines.
The inclusion criteria of studies were as follows: (1) those that reported patients with a history of COVID‐19 vaccination with either the BNT162b2 or mRNA‐1273 vaccine before the presentation of myocarditis; and (2) if the patients were diagnosed with myocarditis with no other identifiable causes based on clinical presentation, elevated levels of cardiac troponin, electrocardiography findings, or cardiac magnetic resonance imaging (CMR); and (3) case report and case series to analyze at individual patient level with sufficient raw data. We excluded cases if they had received any other type of COVID‐19 vaccine or were diagnosed as having pericarditis alone. We further excluded review articles, letters to the editors, abstracts, articles that did not contain sufficient information on the patient characteristics or outcomes, and duplicate cases.
We initially carried out a search on PubMed/Medline, EPub, Scopus, Embase, and Web of Science databases, that include all articles available on patients with COVID‐19 mRNA vaccine‐associated myocarditis published up to August 25, 2021. Our initial search yielded 63 articles. After reviewing individual abstracts and full texts of the articles, we identified 20 studies (12 case reports and 8 case series) that met the inclusion criteria for this systematic review. 10 , 11 , 12 , 13 , 14 , 15 , 16 , 17 , 18 , 19 , 20 , 21 , 22 , 23 , 24 , 25 , 26 , 27 , 28 , 29 In addition, we carried out an additional search in the same databases on September 10, 2021, and added one case series and three case reports. 30 , 31 , 32 , 33 The search terms used are described in detail in Table S2. The detailed selection process is depicted in Figure S1, and the characteristics of individual case studies are shown in Tables S3–S4.
Three reviewers (J.I. Shin, W. Woo, and A.Y. Kim) independently examined the studies, and any disagreement among the reviewers was resolved by consensus. For each eligible case report and case series, we extracted data on the demographic, clinical, and laboratory findings at presentation, types of treatment, clinical course, and outcome.
2.2. Data collection
We identified 24 studies on myocarditis related to immunization with BNT162b2 or mRNA‐1273 COVID‐19 vaccines, and collected data on demographic and clinical characteristics, including information on treatments, outcomes, age, sex, onset of symptoms, pre‐existing conditions, laboratory results, immunologic assays, results of electrocardiography (ECG) and echocardiogram, as well as radiological findings of cardiac magnetic resonance (CMR) imaging, and finally, the length of hospitalization, length of ICU stay, and mortality.
2.3. Statistical analysis
Statistical analyses were performed using the Statistical Package for the Social Sciences (SPSS) for Windows version 25.0 (SPSS Inc., IBM Corporation) and R version 4.0.4 (R Core Team). Basic demographic and clinical information were presented as medians and range for continuous variables and percentage for categorical variables. Continuous variables were compared using the Mann–Whitney U‐test and categorical variables were compared using Fisher's exact test. Spearman's correlation analysis was carried out to determine the relationships between continuous variables, and phi correlation coefficients were calculated to measure the strength of association between categorical variables. We included patients' demographics, clinical presentation, and diagnostic findings, then logistic regression analyses were used to identify independent risk factors for ICU admission, recovery with conservative care, and left ventricular dysfunction. Variables with a p‐value of <0.10 in the univariate analysis were entered into a multivariate analysis and a two‐tailed p‐value of <0.05 was considered significant. Only significant variables in multivariate analyses were listed in the multivariate analyses.
3. RESULTS
3.1. Demographics and clinical characteristics
In regard to age, the 74 patients with myocarditis were 14–70 years old (median age, 17.6) and approximately half of the patients (49.5%) were younger than 20 years. Almost all patients were male (n = 70, 94.6%), and seven patients (9.5%) had underlying medical conditions such as hypertension, diabetes, hyperlipidemia, or endocrinologic disorder. Over two‐thirds (78.3%) of patients received the BNT162b2 vaccine, and most (90.5%) patients presented with myocarditis after the second dose of the vaccine. Patients presented to the hospital from 6 h to 16 days after vaccination, with a median time from vaccination of 3 days. The symptoms presented by these patients are shown in Table S5. Most patients presented with chest pain (95.9%), accompanied with fever (33.8%), dyspnea (21.6%), headache (14.9%), fatigue (10.8%), and chills (5.4%).
ECG findings of myocarditis patients are delineated in Table 1. Over two‐thirds (87.8%) of patients had abnormal ECG findings: ST‐segment (77.0%), T‐wave (16.2%), and PR interval (14.9%). Echocardiography revealed that about a third (31.1%) of patients had left ventricular dysfunction (ejection fraction <55%) and 21 patients had regional wall motion abnormality. In regard to laboratory tests, all 74 patients showed elevated levels of cardiac enzymes, 64 (86.4%) patients had high levels of C‐reactive protein, and 12 (16.2%) patients had a high level of brain natriuretic peptides (BNP), pro‐BNP, or NT‐pro‐BNP. Most patients (79.7%) underwent CMR imaging studies in hospitals, and 40 of 59 patients (67.8%) had CMR findings suggesting myocarditis, which met the original or modified Lake Louise criteria. 34
Table 1.
Characteristics and clinical findings of myocarditis patients after mRNA vaccinations
| Total (n = 74) | mRNA‐1273 (n = 16) | BNT162b2 (n = 58) | ||
|---|---|---|---|---|
| Variables | Number of patients (%) or median (range) | Number of patients (%) or median (range) | Number of patients (%) or median (range) | p value |
| Demographic variables | ||||
| Age, years | 17.6 [14.0–70,0] | 27.0 [20.0–70.0] | 20.0 [14.0–56.0] | 0.008 |
| Male | 70/74 (94.6) | 13/16 (81.2) | 57/58 (98.3) | 0.030 |
| Underlying medical history | 8/74 (10.8) | 4/16 (25.0) | 3/55 (5.5) | 0.036 |
| COVID‐19‐related variables | ||||
| Negative COVID PCR test | 73/73 (100.0) | 15/15 (100.0) | 58/58 (100.0) | NA |
| Previous COVID‐19 infection | 8/45 (17.8) | 1/13 (7.7) | 7/32 (21.9) | 0.405 |
| Presentation after second dose | 67/74 (90.5) | 15/16 (93.8) | 52/58 (89.7) | 1.000 |
| Clinical manifestations | ||||
| Interval after vaccination, days | 3.00[0.25–16.00] | 3.00 [0.25–10.00] | 3.00 [0.25–16.00] | 0.496 |
| Symptoms within 24 h of vaccinationa | 23/33 (69.7) | 10/12 (83.3) | 13/21 (61.9) | 0.259 |
| Symptoms leading to hospitalization | ||||
| Chest pain | 71/74 (95.9) | 14/16 (87.5) | 57/58 (98.3) | 0.116 |
| Chilling | 4/74 (5.4) | 3/16 (18.8) | 1/58 (1.7) | 0.030 |
| Fatigue | 8/74 (10.8) | 1/16 (6.2) | 7/58 (12.1) | 0.678 |
| Fever | 25/74 (33.8) | 5/16 (31.2) | 20/58 (34.5) | 1.000 |
| Headache | 11/74 (14.9) | 1/16 (6.2) | 10/58 (17.2) | 0.437 |
| Dyspnea | 16/74 (21.6) | 5/16 (31.2) | 11/58 (19.0) | 0.315 |
| Electrocardiography | ||||
| Abnormal ECG | 65/74 (87.8) | 13/16 (81.2) | 52/58 (89.7) | 0.396 |
| Non‐sinus rhythm | 6/74 (8.1) | 2/16 (12.5) | 4/58 (6.9) | 0.604 |
| PR interval abnormality | 11/74 (14.9) | 6/16 (37.5) | 5/58 (8.6) | 0.010 |
| ST changes | 57/74 (77.0) | 11/16 (68.8) | 46/58 (79.3) | 0.502 |
| T wave abnormality | 12/74 (16.2) | 0/16 (0.0) | 12/58 (20.7) | 0.058 |
| Echocardiography | ||||
| LVEF, % | 55.0 [27.0–67.5] | 52.0 [27.0–61.0] | 55.0 [37.5–67.5] | 0.016 |
| LV dysfunctionb | 23/74 (31.1) | 9/16 (56.2) | 14/58 (24.1) | 0.030 |
| Pericardial effusion | 7/30 (23.3) | 1/5 (20.0) | 6/25 (24.0) | 1.000 |
| RWMA | 21/43 (48.8) | 9/15 (60.0) | 12/28 (42.9) | 0.347 |
| Laboratory findings | ||||
| Elevated BNPc | 12/22 (54.5) | 2/5 (40.0) | 10/17 (58.8) | 0.624 |
| BNP, pg/ml | 50.0 [22.0–111.0] | 57.2 [22.0–97.0] | 49.0 [42.0–111.0] | 0.905 |
| Pro‐BNP, pg/ml | 428 [149–43 134] | 978 [978] | 402 [149–43 134] | 0.800 |
| NT‐pro‐BNP, pg/ml | 678.5 [571.0–2862.0] | NA | 678.5 [571.0–2862.0] | 0.800 |
| Elevated CRP c | 64/69 (92.8) | 14/14 (100.0) | 50/55 (90.9) | 0.575 |
| CRP, mg/dL | 4.6[0.1–18.1] | 6.32 [0.69–18.1] | 3.78 [0.10–15.5] | 0.082 |
| Leukocytosis c | 11/32 (34.4) | 2/7 (28.6) | 9/25 (36.0) | 1.000 |
| WBC count, per mm3 | 8,855[5000, 17 860] | 10,010 [8, 16, 280, 300] | 8595 [5000, 17860] | 0.196 |
| Elevated cardiac enzymesd | 74/74 (100.0) | 16/16 (100.0) | 58/58 (100.0) | NA |
| MRI findings | ||||
| Myocardial inflammatione | 40/58 (69.0) | 7/11 (63.6) | 33/47 (70.2) | 0.724 |
| Late gadolinium enhancement | 53/57 (93.0) | 10/10 (100.0) | 43/47 (91.5) | 1.000 |
| Hyperemia or scar/necrosis on T1 | 57/59 (96.6) | 11/11 (100.0) | 46/48 (95.8) | 1.000 |
| Myocardial edema on T2 | 40/54 (74.1) | 7/7 (100.0) | 33/47 (70.2) | 0.171 |
| Treatment | ||||
| Conservative care | 26/74 (35.1) | 6/15f (40.0) | 20/58 (34.5) | 0.766 |
| Anti‐inflammatory agents | 40/74 (54.1) | 5/16 (31.2) | 35/58 (60.3) | 0.050 |
| NSAID | 23/74 (31.1) | 2/16 (12.5) | 21/58 (36.2) | 0.125 |
| Colchicine | 15/74 (20.3) | 2/16 (12.5) | 13/58 (22.4) | 0.499 |
| Steroid | 17/74 (23.0) | 3/16 (18.8) | 14/58 (24.1) | 0.750 |
| IV immunoglobulin | 12//74 (16.2) | 0/16 (0.0) | 12/58 (20.7) | 0.058 |
| Heart failure managementg | 12/74 (16.2) | 6/16 (42.9) | 6/58 (10.7) | 0.011 |
| Supplemental oxygen | 2/74 (2.7) | 1/16 (7.1) | 1/58 (1.8) | 0.362 |
| Outcome | ||||
| Complicationh | 4/74 (5.4) | 2/16 (12.5) | 2/58 (3.4) | 0.202 |
| ICU admission | 12/74 (16.2) | 1/16 (6.2) | 11/58 (19.0) | 0.443 |
| Hospital stay, days | 4.0 [1.0–21.0] | 3.0 [2.0–21.0] | 4.0 [1.0–8.0] | 0.295 |
| Hospital stay <4 days | 32/74 (43.2) | 10/12 (83.3) | 22/52 (42.3) | 0.022 |
Abbreviations: BNP, B‐type natriuretic peptide; COVID‐19, coronavirus disease 2019; CRP, C‐reactive protein; ECG, Electrocardiography; ICU, intensive care unit; LVEF, left ventricular ejection fraction; NSAID, nonsteroidal anti‐inflammatory drugs; NT‐proBNP, N‐terminal‐pro‐BNP; RWMA, regional wall motional abnormality; WBC, white blood cells.
Systemic symptoms (fever, chill, myalgia, and generalized body ache) within 24 h after vaccine administration.
LVEF less than 55%.
The normal ranges for selected variables are as follows: BNP <35 pg/ml, proBNP < 100 pg/ml, NT‐proBNP<125 pg/ml; CRP< 0.3 mg/dL, white blood cell count <11 000/mm3, CK <198 IU/L, CK‐MB< 25 IU/L, Troponin<0.04ng/ml, Troponin‐T < 14ng/L, troponin‐I<0.04 ng/ml, hs‐TnT <14 ng/L, hs‐TnI <14 ng/L.
CK, CK‐MB, Troponin, Troponin‐T, Hs‐TnT, and Hs‐TnI.
Using either the original or updated Lake Louise Criteria. 34
One patient's medical treatment was not stated in detail.
Beta‐blocker, diuretics, inotropic, angiotensin‐converting enzyme (ACE) inhibitor, angiotensin‐receptor blocker.
Multiorgan failure (n = 1) and cardiac arrhythmia (n = 3) during hospitalization.
All patients recovered without significant complications. A third (35.1%) of the patients’ symptoms resolved with conservative management. Among the remaining patients, more than half (54.0%) received anti‐inflammatory medications such as nonsteroidal anti‐inflammatory drugs (NSAIDs), colchicine, steroids, or intravenous immunoglobulin. In addition, 16.2% of them were treated with heart failure medications, including beta‐blockers, angiotensin‐converting‐enzyme (ACE) inhibitors/angiotensin‐receptor‐blocker (ARB)s, diuretics, or inotropics (Table S6). About 5% of patients (n = 4) experienced complications, including one major (multiorgan failure) and three minor cases (nonsustained ventricular tachycardia). Twelve patients (16.2%) required ICU care, and about half (43.2%) of the patients were discharged within 4 days.
3.2. Comparison between the BNT162b2 and mRNA‐1273 vaccines
Table 1 shows that myocarditis patients in individuals who received mRNA‐1273 were older than BNT162b2 recipients (median age, 27.0 vs 20.0, p = 0.008). The number of patients with underlying diseases was higher in the mRNA‐1273 group (30.8% vs. 6.0%, p = 0.036). More patients reported chills as a symptom while visiting the hospital in the mRNA‐1273 group (18.8% vs. 1.7%, p < 0.030). Patients who had received BNT162b2 less frequently showed PR interval abnormality in the ECG (8.6% vs. 37.5%, p = 0.010) and less frequent LV dysfunction (24.1% vs. 56.2%, p = 0.030). Other than these, there was no significant difference in terms of laboratory and CMR findings between the groups. Treatment outcomes were also comparable; however, more patients were discharged within 4 days in the mRNA‐1273 group.
3.3. Comparison by age groups
When we divided patients into two groups based on their age, that is, at or over 20 and under 20 years (Table 2), patients under 20 years of age were more likely to present with systemic symptoms such as fever (p = 0.013) or myalgia (p = 0.008). There were more patients with preserved ejection fraction (p = 0.025) and more patients with high levels of BNP (p = 0.004). CMR findings were less prominent in this age group (p = 0.044), and they had fewer patients who received treatment for heart failure (p = 0.003). These were similarly observed in patients who received the BNT162b2 vaccine. Further analysis according to an underlying medical condition, LV function, and first and second dose of vaccines are described in Table S7–S9.
Table 2.
Clinical characteristics of mRNA vaccine‐related myocarditis patients divided by age 20
| Total mRNA vaccines (n = 74) | BNT162b2 (n = 58)a | |||||
|---|---|---|---|---|---|---|
| Age ≥ 20 (N = 40) | Age < 20 (N = 34) | p value | Age ≥ 20 (N = 24) | Age < 20 (N = 34) | p value | |
| Number of patients (%) or median (range) | Number of patients (%) or median (range) | Number of patients (%) or median (range) | Number of patients (%) or median (range) | |||
| Demographic variables | ||||||
| Male | 37/40 (92.5) | 33/34 (97.1) | 0.620 | 24/24 (100.0) | 33/34 (97.1) | 1.000 |
| Underlying medical history | 6/40 (15.0) | 1/34 (2.9) | 0.223 | 2/24 (8.3) | 1/24 (4.2) | 0.564 |
| COVID‐19‐related variables | ||||||
| Previous COVID‐19 infection | 7/33 (21.2) | 1/12 (8.3) | 0.419 | 6/20 (30.0) | 1/12 (8.3) | 0.212 |
| Presentation after second dose | 35/40 (87.5) | 32/34 (94.1) | 0.441 | 20/24 (83.3) | 32/34 (94.1) | 0.220 |
| Type of vaccine | <0.001 | |||||
| mRNA‐1273 | 16/40 (40.0) | 0/40 (0.0) | ||||
| BNT162b2 | 24/40 (60.0) | 34/40 (100.0) | ||||
| Clinical manifestations | ||||||
| Interval after vaccination, days | 3.00 [0.25–16.00] | 2.00 [1.00–6.00] | 0.106 | 3.00 [0.25–16.00] | 2.00 [1.00–6.00] | 0.128 |
| Symptoms within 24 h of vaccinationb | 19/24 (79.2) | 4/9 (44.4) | 0.090 | 9/12 (75.0) | 4/9 (44.4) | 0.203 |
| Symptoms leading to hospitalization | ||||||
| Chest pain | 37/40 (92.5) | 34/34 (100.0) | 0.245 | 23/24 (95.8) | 34/34 (100.0) | 0.414 |
| Fever | 8/40 (20.0) | 17/34 (50.0) | 0.013 | 3/24 (12.5) | 17/34 (50.0) | 0.005 |
| Myalgia | 3/40 (7.5) | 11/34 (32.4) | 0.008 | 1/24 (4.2) | 11/34 (32.4) | 0.010 |
| Electrocardiography | ||||||
| Abnormal ECG | 35/40 (87.5) | 30/34 (88.2) | 1.000 | 22/24 (91.7) | 30/34 (88.2) | 1.000 |
| PR interval abnormality | 11/40 (27.5) | 0/34 (0.0) | 0.001 | 5/24 (20.8) | 0/34 (0.0) | 0.009 |
| Echocardiography | ||||||
| LVEF, % | 52.8 [27.0–67.5] | 56.8 [43.7–64.7] | 0.026 | 54.0 [37.5–67.5] | 56.8 [43.7–64.7] | 0.212 |
| Pericardial effusion | 4/10 (40.0) | 3/20 (15.0) | 0.181 | 3/5 (60.0) | 3/20 (15.0) | 0.070 |
| RWMA | 20/36 (55.6) | 1/7 (14.3) | 0.095 | 11/21 (52.4) | 1/7 (14.3) | 0.184 |
| Laboratory findings | ||||||
| Elevated BNPc | 3/12 (25.0) | 9/10 (90.0) | 0.004 | 1/7 (14.3) | 9/10 (90.0) | 0.004 |
| Elevated CRP | 33/36 (91.7) | 31/33 (93.9) | 1.000 | 19/22 (86.4) | 31/33 (93.9) | 0.379 |
| MRI findings | ||||||
| Myocardial inflammationd | 26/32 (81.3) | 14/26 (53.8) | 0.044 | 19/21 (90.5) | 14/26 (53.8) | 0.010 |
| Late gadolinium enhancement | 31/31 (100.0) | 22/26 (84.6) | 0.038 | 21/21 (100.0) | 22/26 (84.6) | 0.117 |
| Hyperemia or scar/necrosis on T1 | 32/32 (100.0) | 25/27 (92.6) | 0.205 | 21/21 (100.0) | 25/27 (92.6) | 0.497 |
| Myocardial edema on T2 | 26/28 (92.9) | 14/26 (53.8) | 0.002 | 19/21 (90.5) | 14/26 (53.8) | 0.010 |
| Treatment | ||||||
| Conservative care | 12/39* (30.8) | 14/34 (41.2) | 0.463 | 6/24 (25.0) | 14/34 (41.2) | 0.266 |
| Anti‐inflammatory | 21/40 (52.5) | 19/34 (55.9) | 0.818 | 16/24 (66.7) | 19/34 (55.9) | 0.431 |
| Colchicine | 12/40 (30.0) | 3/34 (8.8) | 0.040 | 10/24 (41.7) | 3/34 (8.8) | 0.004 |
| IVIG | 0/40 (0.0) | 12/34 (35.3) | <0.001 | 0/24 (0.0) | 12/34 (35.3) | 0.001 |
| Heart failure management | 11/36 (30.6) | 1/34 (2.9) | 0.003 | 5/22 (22.7) | 1/34 (2.9) | 0.030 |
| Supplement oxygen | 1/36 (2.8) | 1/34 (2.9) | 1.000 | 0/22 (0.0) | 1/34 (2.9) | 1.000 |
| Outcome | ||||||
| Complicatione | 4/40 (10.0) | 0/34 (0.0) | 0.120 | 2/24 (8.3) | 0/34 (0.0) | 0.167 |
| ICU admission | 4/40 (10.0) | 8/34 (23.5) | 0.205 | 3/24 (12.5) | 8/34 (23.5) | 0.333 |
| Hospital stay < 6 days | 22/30 (73.3) | 28/34 (82.4) | 0.546 | 11/18 (61.1) | 28/34 (82.4) | 0.108 |
Abbreviations: BNP, B‐type natriuretic peptide; COVID‐19, coronavirus disease 2019; CRP, C‐reactive protein; ECG, Electrocardiography; ICU, intensive care unit; IVIG, intravenous immunoglobulin; LVEF, left ventricular ejection fraction; NSAID, nonsteroidal anti‐inflammatory drugs; NT‐proBNP, N‐terminal‐pro‐BNP; RWMA, regional wall motional abnormality; WBC, white blood cells.
mRNA‐1273 vaccine was not administered under age 20.
Systemic symptoms (fever, chill, myalgia, and generalized body ache) within 24 h after vaccine administration.
Included in this category if patient has elevated levels of BNP, proBNP, or NT‐proBNP.
Using either the original or updated Lake Louise Criteria.34
Multiorgan failure (n = 1) and cardiac arrhythmia (n = 3) during hospitalization.
3.4. Risk factor analysis for treatment outcome
A third (35.1%) of the patients recovered without any medical treatment such as anti‐inflammatory agents or heart failure medications (Table 3). They had more preserved LV function (p = 0.015) and less significant CMR findings on T2 images (p = 0.033). In the logistic regression analyses for recovery with conservative care, previous COVID‐19 infection, normal ECG, preserved ejection fraction, and positive CMR findings were significant in the univariate analyses. Finally, previous COVID‐19 infection was found to be the only significant factor (OR, 25.0; 95% CI, 1.82–343.0; p = 0.016) in multivariate analysis (Table 4).
Table 3.
Clinical characteristics of mRNA vaccine‐related myocarditis patients according to recovery course
| Total mRNA vaccines (n = 74) | BNT162b2 (n = 58)a | |||||
|---|---|---|---|---|---|---|
| Medical treatment (n = 47) | Conservative careb (n = 26) | p value | Medical treatment (n = 38) | Conservative care b (n = 20) | p value | |
| Number of patients (%) or median (range) | Number of patients (%) or median (range) | Number of patients (%) or median (range) | Number of patients (%) or median (range) | |||
| Demographic variables | ||||||
| Age, years | 22.0 [14.0–67.0] | 23.5 [16.2–56.0] | 0.668 | 20.0 [14.0–45.0] | 20.8 [16.2–56.0] | 0.587 |
| Male | 46/47 (97.9) | 24/26 (92.3) | 0.287 | 38/38 (100.0) | 19/20 (95.0) | 0.345 |
| Underlying disease | 6/46 (13.0) | 0/16 (0.0) | 0.325 | 3/37 (8.1) | 0/13 (0.0) | 0.558 |
| COVID‐19‐related variables | ||||||
| Previous COVID‐19 infection | 5/37 (13.5) | 3/7 (42.9) | 0.100 | 4/28 (14.3) | 3/4 (75.0) | 0.025 |
| Presentation after second dose | 43/47 (91.5) | 23/26 (88.5) | 0.694 | 35/38 (92.1) | 17/20 (85.0) | 0.405 |
| Type of vaccine | 0.766 | |||||
| mRNA‐1273 | 9/47 (19.1) | 6/26 (23.1) | ||||
| BNT162b2 | 38/47 (80.9) | 20/26 (76.9) | ||||
| Clinical manifestations | ||||||
| Interval after vaccination, days | 3.00 [0.25–16.00] | 3.00 [1.00–6.00] | 0.834 | 2.00 [0.25–16.00] | 3.00 [1.00–6.00] | 0.609 |
| Symptoms with 24 h of vaccinationc | 19/25 (76.0) | 4/7 (57.1) | 0.370 | 11/17 (64.7) | 2/4 (50.0) | 0.618 |
| Chest pain | 45/47 (95.7) | 25/26 (96.2) | 1.000 | 38/38 (100.0) | 19/20 (95.0) | 0.345 |
| Fatigue | 7/47 (14.9) | 1/26 (3.8) | 0.245 | 6/38 (15.8) | 1/20 (5.0) | 0.403 |
| Fever | 16/47 (34.0) | 9/26 (34.6) | 1.000 | 12/38 (31.6) | 8/20 (40.0) | 0.570 |
| Nausea | 7/47 (14.9) | 1/26 (3.8) | 0.245 | 5/38 (13.2) | 1/20 (5.0) | 0.653 |
| Electrocardiography | ||||||
| Abnormal ECG | 44/47 (93.6) | 20/26 (76.9) | 0.061 | 37/38 (97.4) | 15/20 (75.0) | 0.016 |
| Non‐sinus rhythm | 6/47 (12.8) | 0/26 (0.0) | 0.083 | 4/38 (10.5) | 0/20 (0.0) | 0.288 |
| ST changes | 38/47 (80.9) | 18/26 (69.2) | 0.386 | 33/38 (86.8) | 13/20 (65.0) | 0.086 |
| Echocardiography | ||||||
| LV dysfunctiond | 19/47 (40.4) | 3/26 (11.5) | 0.015 | 14/38 (36.8) | 0/20 (0.0) | 0.001 |
| RWMA | 15/32 (46.9) | 5/10 (50.0) | 1.000 | 11/24 (45.8) | 1/4 (25.0) | 0.613 |
| Laboratory findings | ||||||
| Elevated BNPe | 8/17 (47.1) | 3/4 (75.0) | 0.586 | 7/13 (53.8) | 3/4 (75.0) | 0.603 |
| Elevated CRP | 40/44 (90.9) | 24/25 (96.0) | 0.646 | 32/36 (88.9) | 18/19 (94.7) | 0.649 |
| MRI findings | ||||||
| Late gadolinium enhancement | 40/41 (97.6) | 12/15 (80.0) | 0.055 | 34/35 (97.1) | 9/12 (75.0) | 0.046 |
| Hyperemia or myocardial fibrosis on T1 | 42/43 (97.7) | 14/15 (93.3) | 0.454 | 35/36 (97.2) | 11/12 (91.7) | 0.441 |
| Myocardial edema on T2 | 32/39 (82.1) | 7/14 (50.0) | 0.033 | 28/35 (80.0) | 5/12 (41.7) | 0.025 |
| Outcome | ||||||
| Complicationf | 3/47 (6.4) | 1/26 (3.8) | 1.000 | 2/38 (5.3) | 0/20 (0.0) | 0.540 |
| ICU admission | 12/47 (25.5) | 0/26 (0.0) | 0.006 | 11/38 (28.9) | 0/20 (0.0) | 0.011 |
| Hospital stay < 4 days | 17/40 (42.5) | 14/23 (60.9) | 0.196 | 12/33 (36.4) | 10/19 (52.6) | 0.382 |
Abbreviations: BNP, B‐type natriuretic peptide; COVID‐19, coronavirus disease 2019; CRP, C‐reactive protein; ECG, Electrocardiography; ICU, intensive care unit; IVIG, intravenous immunoglobulin; LVEF, left ventricular ejection fraction; NSAID, nonsteroidal anti‐inflammatory drugs; NT‐proBNP, N‐terminal‐pro‐BNP; RWMA, regional wall motional abnormality.
mRNA‐1273 vaccine did not show any significant difference in parameters between spontaneous recovery and medical treatment groups.
Patients who recovered without medical treatments such as anti‐inflammatory agents or heart failure treatment.
Systemic symptoms (fever, chill, myalgia, and generalized body ache) within 24 h after vaccine administration.
LVEF less than 55%.
Included in this category if patient has elevated levels of BNP, proBNP, or NT‐proBNP.
Multiorgan failure (n = 1) and cardiac arrhythmia (n = 3) during hospitalization.
Table 4.
Risk factor analysis according to clinical presentation and outcome among mRNA vaccines related myocarditis patients
| Fisher's exact test | Univariate logistic regression | Multivariate logistic regression | ||||||
|---|---|---|---|---|---|---|---|---|
| Factor | Event | Total | p‐value | OR (95% CI) | p value | OR (95% CI) | p value | |
| 1. ICU admission | ||||||||
| Nausea | 8 | 66 | 0.020* | 7.25 (1.51–34.90) | 0.013* | |||
| No nausea | 4 | 8 | Ref. | |||||
| Vomiting | 9 | 70 | 0.012* | 20.30 (1.90–217.00) | 0.013* | 20.30 (1.90–217.00) | 0.013 * | |
| No vomiting | 3 | 1 | Ref. | |||||
| 2. LV dysfunctiona | ||||||||
| Age < 20 | 6 | 34 | 0.025* | 0.29 (0.10–0.86) | 0.025* | |||
| Age ≥ 20 | 17 | 40 | Ref. | |||||
| BNT162b2 vaccine | 14 | 44 | 0.030* | 0.25 (0.08–0.79) | 0.018* | |||
| mRNA‐1273 vaccine | 9 | 7 | Ref. | |||||
| No leukocytosis | 5 | 21 | 0.123 | 0.26 (0.06–1.23) | 0.090 | |||
| Leukocytosis | 6 | 11 | Ref. | |||||
| 3. Recovery with conservative careb | ||||||||
| Previous COVID‐19 history | 3 | 8 | 0.100 | 4.80 (0.82–28.20) | 0.082 | 25.00 (1.82–343.00) | 0.016 * | |
| No COVID‐19 history | 4 | 36 | Ref. | |||||
| Normal ECG | 6 | 9 | 0.061 | 4.40 (1.00–19.40) | 0.050 | |||
| Abnormal ECG | 20 | 64 | Ref. | |||||
| Preserved LVEF | 23 | 51 | 0.015* | 5.20 (1.37–19.80) | 0.016* | |||
| Low LVEF | 3 | 22 | Ref. | |||||
| Myocardial edema in T2MR | 7 | 39 | 0.033* | 0.219 (0.058–0.826) | 0.025* | |||
| No Myocardial edema in T2MR | 7 | 14 | Ref. | |||||
Abbreviations: COVID‐19, coronavirus disease 2019; ECG, Electrocardiography; ICU, intensive care unit; LV, left ventricle; LVEF, left ventricular ejection fraction; T2MR: T2 image on cardiac magnetic resonance imaging.
p < .05.
LVEF less than 55%.
Patients who reovered without medical treatments such as anti‐inflammatory agents or heart failure treatment.
Gastrointestinal symptoms, such as anorexia (p = 0.024), nausea (p = 0.020), or vomiting (p = 0.012), were more prevalent in 12 patients (16.2%) who required intensive care (Table S10). They required additional medical treatments (p = 0.006), and more patients stayed for over 6 days in the hospital (p = 0.019). In the multivariate logistic regression analyses, patients who experienced vomiting as a symptom were significantly related to ICU care (OR, 20.3; 95% CI, 1.90–217.0; p = 0.013) (Table 4).
3.5. Correlation among key clinical findings
Several factors showed significant correlations when we analyzed the Phi coefficient among the clinical findings (Figure 1). Positive correlations were found between the younger age group and preserved ejection fraction among individuals who received the BNP162b2 vaccine. ICU admission also showed positive correlations with elevated BNP levels and anti‐inflammatory treatment. On the contrary, significant negative relationships were found between myalgia and CMR findings, COVID‐19 history, and the second dose of the vaccine. Other meaningful correlations among variables are also described in Figure 1.
Figure 1.
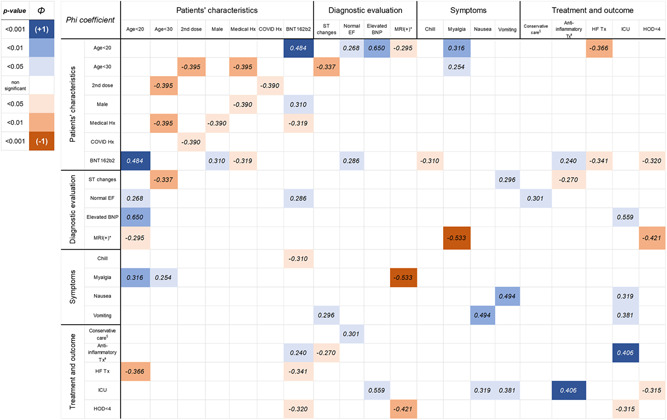
Correlation among key clinical characteristics and diagnostic findings with Phi(Φ) coefficient Tx, treatment; EF, ejection fraction; Hx, history; BNP, B‐type natriuretic peptide; HOD, hospital stay. *Cardiac MRI findings satisfying original or modified Lake Louise criteria. 34 §Conservative care: patients who recovered without administration of anti‐inflammatory agents or cardiovascular medications. ◻Treatment including colchicine, nonsteroidal anti‐inflammatory drugs, steroid, or intravenous immunoglobulin
3.6. COVID‐19 myocarditis versus vaccine‐induced myocarditis
Figure 2 describes the treatment and clinical outcomes between COVID‐19‐related myocarditis and COVID‐19 mRNA vaccine‐related myocarditis patients. We reviewed the data of 42 COVID‐19‐related myocarditis patients from two systematic reviews 35 , 36 and compared the outcomes with 74 patients in this review. There was no difference in the proportion of patients who received steroid, colchicine, or IV immunoglobulin. However, the number of patients who experienced complications or required intensive care was significantly lower among the mRNA vaccine‐related myocarditis patients. Furthermore, there was no mortality reported among the mRNA vaccine‐related myocarditis patients, and more patients recovered without myocarditis‐specific medical treatments.
Figure 2.
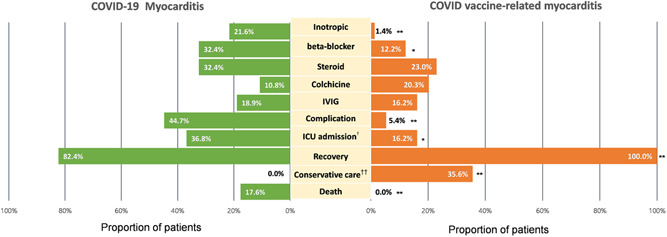
Treatment outcome of myocarditis patients between COVID‐19 induced and COVID vaccine related *p < 0.05, **p < 0.001. †It accounts for cases that needed intubation, mechanical ventilation, or ECMO support. ††It included patients who did not receive anti‐inflammatory agents, steroids, IV immunoglobulin, cardiovascular medications We compared the pooled data of 42 patients in two systematic reviews 34 , 35 of case reports about myocarditis due to COVID‐19 infection and our data of myocarditis patients who developed after mRNA vaccinations
4. DISCUSSION
A case report on myocarditis linked to the COVID‐19 mRNA vaccines was first published in March 2021, 33 and ever since it has been a challenge to understand this emerging new adverse event of the mRNA vaccine owing to lack of incorporated data. To the best of our knowledge, this systematic review is the first to analyze such a large sample and provides information for understanding the clinical features of this rare adverse event. This systematic review summarizes 74 cases of myocarditis that received the BNT162b2 or mRNA‐1273 vaccines to analyze the clinical manifestations, treatment modalities, outcomes, and prognostic factors associated with adverse outcomes. Notably, our study compared clinical characteristics, diagnostic findings, and outcomes based on the type of mRNA vaccines and the patients' age. This review provides clinicians with a comprehensive understanding of this rare adverse event.
It is generally accepted that the acute onset of vaccine‐associated myocarditis is attributable to allergic/hypersensitivity reactions as observed in other vaccines. 37 , 38 For myocarditis after COVID‐19 vaccination, however, there have been additional plausible hypotheses. In previous reports, these patients did not have eosinophilia or immune complex deposition in laboratory studies, 10 , 11 , 13 , 19 , 21 , 26 , 30 , 31 , 33 , 39 in contrast to serum sickness or hypersensitivity reactions. Though myocardial biopsies were performed in small patients, they also did not have eosinophilic infiltration. 19 , 28 Several proposed mechanisms alternative to allergic/hypersensitivity reaction for this rare adverse event are following: (1) elevated innate immune response against modified nucleoside of vaccines in people with genetic predisposition; 39 , 40 , 41 (2) molecular mimicry between self‐antigen and spike protein formed by vaccines, which results in the dysregulated activation of immunologic pathways. 39 , 41 , 42
Several studies have compared adverse events based on the type of vaccines. Meo et al. reported a lower rate of adverse events after receiving BNT162b2 based on the patient's symptoms and anaphylaxis reports, as compared to mRNA‐1273. 43 Despite this, there has been no study that compared myocarditis as an adverse event between the two mRNA‐based vaccines. In laboratory reports, IgG levels to the SARS‐CoV‐2 spike receptor‐binding domain were lower in recipients who received BNT162b2, compared with mRNA‐1273, after the first and second doses. 44 As it was stated earlier about the mechanism of myocarditis in the aspects of molecular mimicry, higher antibody levels in mRNA‐1273 recipients could be attributable to more systemic symptoms and an advanced state of myocarditis‐related immunologic reaction. This laboratory result could support our finding that chills and LV dysfunction were more prevalent in mRNA‐1273 recipients. 45 However, due to the limited number of mRNA‐1273 myocarditis cases and the nonavailability of laboratory data on the levels of antibodies, it is currently challenging to make definitive conclusions regarding an association between myocarditis and antibody levels. Also, contrary to clinical symptoms and echocardiography findings, more patients who received mRNA‐1273 were discharged within four days compared to those who received BNT162b2. Further studies are necessary to draw conclusions and understand the difference between the two vaccines regarding myocarditis as an adverse event.
In addition, young patients were more likely to experience systemic symptoms such as fever and myalgia. Richards et al. evaluated humoral antibody levels after the primary immunization, and the younger age group showed significantly higher antibody response than older individuals. 45 A possible explanation could be a more potent immune response in younger patients, which can also explain the higher rate of side effects to the vaccines in this age group. 46 However, this age group (<20 years) reported fewer cases of LV dysfunction, and the number of patients with prominent myocardial inflammation findings in the CMR was lower than that observed in older patients in our review. Due to insufficient reports on adolescents who received mRNA vaccines, further analyses for young individuals are necessary.
In agreement with previous reports on these patients' relatively favorable clinical courses, 5 , 47 symptoms of all 74 patients in this review resolved, and nearly a third (31.0%) of them recovered with conservative treatment. In contrast to these findings, several reports have described fatal clinical courses in vaccine‐related myocarditis patients in databases. 8 , 48 , 49 , 50 Thus, patients with worse outcomes might not have been reported, and this study's bias in data collection should also be considered. Additionally, this review does not represent the long‐term prognosis of these patients. As general myocarditis patients experience fatal cardiac‐related outcomes after recovering from the first event, 51 , 52 , 53 clinicians should be cautious to determine any conclusion in terms of long‐term prognosis.
Meanwhile, clinical presentations accompanied with vomiting were highly related to ICU admission in the risk factor analysis. Though the relationship between myocarditis and GI symptoms is unclear, it could be intuited from the significance of gastrointestinal symptoms in heart failure patients, which is cardiointestinal syndrome. 54 , 55 The gastrointestinal system acts as a venous reservoir 56 and a crucial immunologic barrier representing the largest mass of lymphoid tissue in the body. 57 As the ejection fraction reduces in myocarditis patients, the capacity of the splanchnic vein also decreases and shifts fluids out of the splanchnic system, thereby increasing the effective circulating volume. Due to hypoperfusion and edema of the gastrointestinal system, gut permeability increases and results in the translocation of bacterial or lipopolysaccharide. 58 Then, the elevated level of endotoxin and cytokines finally results in high immunologic reactions and a worse prognosis. 54 , 55 , 59 Additionally, several reports indicate that a history of gastrointestinal symptoms conferred a greater risk of mortality in pediatric myocarditis patients. 60 , 61 , 62 Therefore, these possible mechanisms could put myocarditis patients with gastrointestinal symptoms after mRNA vaccination at higher risk than others. Further immunologic studies and further clinical analysis would be necessary to find the causal relationship and the pathogenesis of gastrointestinal symptoms in these patients.
Many patients received anti‐inflammatory agents such as NSAID, colchicine, steroid, and intravenous immunoglobulin. Though the reasons for selecting specific drugs were not described in the included studies, the use of drug regimens was in line with the current medical knowledge of general myocarditis, 63 in contrast to SARS‐CoV‐2 related myocarditis patients who received several experimental drugs. 35 Notably, symptoms of one‐third (35.1%) of the patients resolved with conservative treatment, and previous SARS‐CoV‐2 infection was related to this favorable recovery process. Patients who recovered from earlier SARS‐CoV‐2 exposure may have an immune system that is primed from it. Patients with previous exposure to COVID‐19 reported higher SARS‐CoV‐2 spike IgG titers, before and after the first and second dose of an mRNA vaccine, compared to those without previous infection. 64 Also, the function of B‐cells specific to receptor binding domains remained unchanged at 6.2 months after infection, 65 and the plasma neutralizing activity and relative numbers of receptor binding domain‐specific memory B cells of individuals who had recovered from natural infection was higher and equivalent to those who were vaccinated. 44 Therefore, these immunologic conditions in patients who had recovered from SARS‐CoV‐2 could more favorably facilitate recovery from myocarditis after receiving an mRNA vaccine.
Meanwhile, myocarditis related to SARS‐CoV‐2 infection has been reported since the beginning of the pandemic. 66 , 67 , 68 Multiple studies have reported the prevalence of cardiac complications in adults after being diagnosed with COVID‐19, which included heart failure (23%–33.3%), myocardial injury/myocarditis (8%–27.8%), arrhythmia (16.7%), and thromboembolism (31%–40%). Among these, high mortality rates (51%–97%) have been described in several cases series. 35 , 69 Starekova et al. found that there was a low prevalence of myocarditis (1.4%) among student‐athletes recovering from COVID‐19 with none, mild, to moderate symptoms by CMR. 70 Recently published data from the health care organization in Israel estimated the incidence of myocarditis due to COVID‐19 infection as 11.0 cases per 100 000 persons (95% CI, 5.6–15.8), and the incidence of myocarditis following the BNT1621b vaccine was 2.7 cases per 100 000 persons (95% CI, 1.0–4.6). 71 The systematic reviews of 42 myocarditis cases related to COVID‐19 infection revealed a high mortality rate and severe complications, 35 , 36 compared to the present review's findings. Although the incidence of myocarditis in the vaccinated population is higher than in unvaccinated individuals, the risk of myocarditis due to COVID‐19 and its fatal outcome is much lower among vaccinated people. Moreover, infection with SARS‐CoV‐2 has more adverse events beyond myocarditis, and thus, it is necessary to encourage the public to get vaccinated.
The present systematic review has several limitations. First, it is difficult to generalize the study findings due to rapidly evolving medical knowledge about SARS‐CoV‐2 and its mRNA vaccines. This rare adverse event could be attributable to genetic predispositions of a specific population, as we mentioned the possible mechanism forehead. Furthermore, as we reviewed case reports, information about myocarditis related to mRNA vaccines has been updated continuously, and the eligibility of mRNA vaccination has also evolved. Cautious interpretation of our data is necessary based on this ever‐changing medical environment. Second, the direct comparison of two mRNA vaccines should be carefully elucidated. Due to different vaccine eligibility criteria and the scarcity of myocarditis, we could not correct confounding factors such as demographic factors, locoregional policy in COVID‐19 vaccination, and other accountable factors. Third, the data of case reports may have been incomplete. Due to insufficient data on continuous variables, many were classified as categorical variables, possibly limiting the strength of our statistical analysis. Third, information about clinical situations is widely varied. In terms of patient care, all cases did not specify the reason for medical decisions related to medications, ICU admission, and follow‐up plans. Therefore, the risk of bias in data interpretation remains elevated. Further large‐scale and multicenter studies addressing mRNA vaccine‐associated myocarditis are necessary.
5. CONCLUSION
This systematic review summarizes clinical features, diagnostic findings, management, and myocarditis outcomes associated with mRNA vaccines. The risk of fatality in myocardial inflammation related to mRNA vaccines seems to be very low, and a significant proportion of patients recovered with conservative management. Previous SARS‐CoV‐2 infection was related to recovery with conservative care, and patients with nausea were more likely to require ICU admission. Otherwise, both BNT162b2 and mRNA‐1273 vaccines do not demonstrate a difference in the clinical outcome of myocarditis patients. However, there are some variations concerning clinical manifestation and diagnostic findings. It is crucial to incorporate and analyze large‐scale multicenter data to understand clinical characteristics of mRNA vaccine‐related myocarditis to efficiently educate the public.
CONFLICT OF INTERESTS
The authors declare that there are no conflict of interests.
ETHICS STATEMENT
Ethical approval was not required because this study was a pooled analysis of published articles.
AUTHOR CONTRIBUTIONS
Wongi Woo, Ah Young Kim, Seung Won Lee, Dong Keon Yon, SS and Jae Il Shin designed this study. Wongi Woo, Ah Young Kim, and Jae Il Shin collected the data, and Wongi Woo, Ah Young Kim, Seung Won Lee, Dong Keon Yon, SS and Jae Il Shin performed the statistical analysis. Wongi Woo, Ah Young Kim, SS, and Jae Il Shin wrote the first draft of the manuscript. All authors had full access to all the study data. All authors reviewed, wrote, and approved the final version. The corresponding authors had final responsibility for the decision to submit for publication.
Supporting information
Supporting information.
ACKNOWLEDGEMENTS
WW and AYK are spouses. However, both equally contributed to conceptualization, data curation, analysis, and writing the manuscript. This study did not receive any specific grant from funding agencies in the public, commercial, or not‐for‐profit sectors.
Woo W, Kim AY, Yon DK, et al. Clinical characteristics and prognostic factors of myocarditis associated with the mRNA COVID‐19 vaccine. J Med Virol. 2022;94:1566‐1580. 10.1002/jmv.27501
Wongi Woo, Ah Y. Kim, Dong K. Yon, Seung W. Lee are co‐first authors.
Contributor Information
Sungsoo Lee, Email: chestlee@yuhs.ac.
Jae Il Shin, Email: shinji@yuhs.ac.
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available from the corresponding author upon reasonable request.
REFERENCES
- 1. COVID‐19 Map ‐ Johns Hopkins Coronavirus Resource Center . Accessed September 12, 2021. https://coronavirus.jhu.edu/map.html
- 2. Voysey M, Clemens SAC, Madhi SA, et al. Safety and efficacy of the ChAdOx1 nCoV‐19 vaccine (AZD1222) against SARS‐CoV‐2: an interim analysis of four randomised controlled trials in Brazil, South Africa, and the UK. Lancet. 2021;397(10269):99‐111. 10.1016/S0140-6736(20)32661-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Polack FP, Thomas SJ, Kitchin N, et al. Safety and efficacy of the BNT162b2 mRNA Covid‐19 vaccine. N Engl J Med. 2020;383(27):2603‐2615. 10.1056/NEJMoa2034577 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Baden LR, El Sahly HM, Essink B, et al. Efficacy and safety of the mRNA‐1273 SARS‐CoV‐2 vaccine. N Engl J Med. 2021;384(5):403‐416. 10.1056/NEJMoa2035389 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Montgomery J, Ryan M, Engler R, et al. Myocarditis following immunization With mRNA COVID‐19 Vaccines In Members of the US Military. JAMA Cardiol. 2021;6:1202‐1206. 10.1001/jamacardio.2021.2833 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. VAERS ‐ Data . Accessed September 18, 2021. https://vaers.hhs.gov/data.html
- 7. BUCKINGHAM L. Safety of COVID‐19 vaccines. European Medicines Agency. 2021. Accessed September 13, 2021. https://www.ema.europa.eu/en/human-regulatory/overview/public-health-threats/coronavirus-disease-covid-19/treatments-vaccines/vaccines-covid-19/safety-covid-19-vaccines [Google Scholar]
- 8. European database of suspected adverse drug reaction reports ‐ Search . Accessed September 13, 2021. https://www.adrreports.eu/en/search_subst.html
- 9. Moher D, Shamseer L, Clarke M, et al. Preferred reporting items for systematic review and meta‐analysis protocols (PRISMA‐P) 2015 statement. Syst Rev. 2015;4:1. 10.1186/2046-4053-4-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Abu Mouch S, Roguin A, Hellou E, et al. Myocarditis following COVID‐19 mRNA vaccination. Vaccine. 2021;39(29):3790‐3793. 10.1016/j.vaccine.2021.05.087 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Bautista García J, Peña Ortega P, Bonilla Fernández JA, Cárdenes León A, Ramírez Burgos L, Caballero Dorta E. Acute myocarditis after administration of the BNT162b2 vaccine against COVID‐19. Rev Esp Cardiol (Engl Ed). 2021;74(9):812‐814. 10.1016/j.rec.2021.04.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Cereda A, Conca C, Barbieri L, et al. Acute myocarditis after the second dose of SARS‐CoV‐2 vaccine: serendipity or atypical causal relationship? Anatol J Cardiol. 2021;25(7):522‐523. 10.5152/AnatolJCardiol.2021.99 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. D'angelo T, Cattafi A, Carerj ML, et al. Myocarditis after SARS‐CoV‐2 vaccination: a vaccine‐induced reaction. Can J Cardiol. Published online June 9, 2021;S0828‐282X(21):00286‐5. 10.1016/j.cjca.2021.05.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Deb A, Abdelmalek J, Iwuji K, Nugent K. Acute myocardial injury following COVID‐19 vaccination: a case report and review of current evidence from vaccine adverse events reporting system database. J Prim Care Community Health. 2021;12:21501327211029230. 10.1177/21501327211029230 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Dionne A, Sperotto F, Chamberlain S, et al. Association of myocarditis with BNT162b2 messenger RNA COVID‐19 vaccine in a case series of children. JAMA Cardiol. Published online August 10, 2021. 10.1001/jamacardio.2021.3471 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Hasnie AA, Hasnie UA, Patel N, et al. Perimyocarditis following first dose of the mRNA‐1273 SARS‐CoV‐2 (Moderna) vaccine in a healthy young male: a case report. BMC Cardiovasc Disord. 2021;21(1):375. 10.1186/s12872-021-02183-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Khogali F, Abdelrahman R. Unusual presentation of acute perimyocarditis following SARS‐COV‐2 mRNA‐1237 Moderna vaccination. Cureus. 2021;13(7):e16590. 10.7759/cureus.16590 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. King WW, Petersen MR, Matar RM, Budweg JB, Cuervo Pardo L, Petersen JW. Myocarditis following mRNA vaccination against SARS‐CoV‐2, a case series. Am Heart J Plus. 2021;8:100042. 10.1016/j.ahjo.2021.100042 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Larson KF, Ammirati E, Adler ED, et al. Myocarditis After BNT162b2 and mRNA‐1273 Vaccination. Circulation. 2021;144(6):506‐508. 10.1161/CIRCULATIONAHA.121.055913 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Mansour J, Short RG, Bhalla S, et al. Acute myocarditis after a second dose of the mRNA COVID‐19 vaccine: a report of two cases. Clin Imaging. 2021;78:247‐249. 10.1016/j.clinimag.2021.06.019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Marshall M, Ferguson ID, Lewis P, et al. Symptomatic acute myocarditis in 7 adolescents after Pfizer‐BioNTech COVID‐19 vaccination. Pediatrics. Published online September 1, 2021;148, 10.1542/peds.2021-052478 [DOI] [PubMed] [Google Scholar]
- 22. Kim IC, Kim H, Lee HJ, Kim JY, Kim JY. Cardiac imaging of acute myocarditis following COVID‐19 mRNA vaccination. J Korean Med Sci. 2021;36(32):e229. 10.3346/jkms.2021.36.e229 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Minocha PK, Better D, Singh RK, Hoque T. Recurrence of acute myocarditis temporally associated with receipt of the mRNA coronavirus disease 2019 (COVID‐19) vaccine in a male adolescent. J Pediatr. Published online June 22, 2021;S0022‐3476(21):00617‐0061. 10.1016/j.jpeds.2021.06.035 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Muthukumar A, Narasimhan M, Li QZ, et al. In‐depth evaluation of a case of presumed myocarditis after the second dose of COVID‐19 mRNA vaccine. Circulation. 2021;144(6):487‐498. 10.1161/CIRCULATIONAHA.121.056038 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Patrignani A, Schicchi N, Calcagnoli F, et al. Acute myocarditis following Comirnaty vaccination in a healthy man with previous SARS‐CoV‐2 infection. Radiol Case Rep. 2021;16(11):3321‐3325. 10.1016/j.radcr.2021.07.082 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Rosner CM, Genovese L, Tehrani BN, et al. Myocarditis temporally associated With COVID‐19 vaccination. Circulation. 2021;144(6):502‐505. 10.1161/CIRCULATIONAHA.121.055891 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Singh B, Kaur P, Cedeno L, et al. COVID‐19 mRNA vaccine and myocarditis. Eur J Case Rep Intern Med. 2021;8(7):002681. 10.12890/2021_002681 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Snapiri O, Rosenberg Danziger C, Shirman N, et al. Transient cardiac injury in adolescents receiving the BNT162b2 mRNA COVID‐19 vaccine. Pediatr Infect Dis J. 2021;40:360. 10.1097/INF.0000000000003235 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Watkins K, Griffin G, Septaric K, Simon EL. Myocarditis after BNT162b2 vaccination in a healthy male. Am J Emerg Med. Published online June 29, 2021;S0735‐6757(21):00536‐2. 10.1016/j.ajem.2021.06.051 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. McLean K, Johnson TJ. Myopericarditis in a previously healthy adolescent male following COVID‐19 vaccination: a case report. Acad Emerg Med. 2021;28(8):918‐921. 10.1111/acem.14322 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Albert E, Aurigemma G, Saucedo J, Gerson DS. Myocarditis following COVID‐19 vaccination. Radiol Case Rep. 2021;16(8):2142‐2145. 10.1016/j.radcr.2021.05.033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Kim HW, Jenista ER, Wendell DC, et al. Patients with acute myocarditis following mRNA COVID‐19 vaccination. JAMA Cardiol. 2021;6:1196‐1201. 10.1001/jamacardio.2021.2828 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Ammirati E, Cavalotti C, Milazzo A, et al. Temporal relation between second dose BNT162b2 mRNA Covid‐19 vaccine and cardiac involvement in a patient with previous SARS‐COV‐2 infection. Int J Cardiol Heart Vasc. 2021;34:100774. 10.1016/j.ijcha.2021.100774 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Ferreira VM, Schulz‐Menger J, Holmvang G, et al. Cardiovascular magnetic resonance in nonischemic myocardial inflammation: expert recommendations. J Am Coll Cardiol. 2018;72(24):3158‐3176. 10.1016/j.jacc.2018.09.072 [DOI] [PubMed] [Google Scholar]
- 35. Castiello T, Georgiopoulos G, Finocchiaro G, et al. COVID‐19 and myocarditis: a systematic review and overview of current challenges. Heart Fail Rev. Published online March 24, 2021. 10.1007/s10741-021-10087-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Rathore SS, Rojas GA, Sondhi M, et al. Myocarditis associated with Covid‐19 disease: a systematic review of published case reports and case series. Int J Clin Pract. 2021;75:e14470. 10.1111/ijcp.14470 [DOI] [PubMed] [Google Scholar]
- 37. Yamamoto H, Hashimoto T, Ohta‐Ogo K, et al. A case of biopsy‐proven eosinophilic myocarditis related to tetanus toxoid immunization. Cardiovasc Pathol. 2018;37:54‐57. 10.1016/j.carpath.2018.10.003 [DOI] [PubMed] [Google Scholar]
- 38. Mei R, Raschi E, Forcesi E, Diemberger I, De Ponti F, Poluzzi E. Myocarditis and pericarditis after immunization: gaining insights through the vaccine adverse event reporting system. Int J Cardiol. 2018;273:183‐186. 10.1016/j.ijcard.2018.09.054 [DOI] [PubMed] [Google Scholar]
- 39. Bozkurt B, Kamat I, Hotez PJ. Myocarditis With COVID‐19 mRNA Vaccines. Circulation. 2021;144(6):471‐484. 10.1161/CIRCULATIONAHA.121.056135 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Karikó K, Buckstein M, Ni H, Weissman D. Suppression of RNA recognition by Toll‐like receptors: the impact of nucleoside modification and the evolutionary origin of RNA. Immunity. 2005;23(2):165‐175. 10.1016/j.immuni.2005.06.008 [DOI] [PubMed] [Google Scholar]
- 41. Caso F, Costa L, Ruscitti P, et al. Could Sars‐coronavirus‐2 trigger autoimmune and/or autoinflammatory mechanisms in genetically predisposed subjects? Autoimmun Rev. 2020;19(5):102524. 10.1016/j.autrev.2020.102524 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Vojdani A, Kharrazian D. Potential antigenic cross‐reactivity between SARS‐CoV‐2 and human tissue with a possible link to an increase in autoimmune diseases. Clin Immunol. 2020;217:108480. 10.1016/j.clim.2020.108480 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Meo SA, Bukhari IA, Akram J, Meo AS, Klonoff DC. COVID‐19 vaccines: comparison of biological, pharmacological characteristics and adverse effects of Pfizer/BioNTech and Moderna Vaccines. Eur Rev Med Pharmacol Sci. 2021;25(3):1663‐1669. 10.26355/eurrev_202102_24877 [DOI] [PubMed] [Google Scholar]
- 44. Wang Z, Schmidt F, Weisblum Y, et al. mRNA vaccine‐elicited antibodies to SARS‐CoV‐2 and circulating variants. Nature. 2021;592(7855):616‐622. 10.1038/s41586-021-03324-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Richards NE, Keshavarz B, Workman LJ, Nelson MR, Platts‐Mills TAE, Wilson JM. Comparison of SARS‐CoV‐2 antibody response by age among recipients of the BNT162b2 vs the mRNA‐1273 vaccine. JAMA Netw Open. 2021;4(9):e2124331. 10.1001/jamanetworkopen.2021.24331 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Li J, Hui A, Zhang X, et al. Safety and immunogenicity of the SARS‐CoV‐2 BNT162b1 mRNA vaccine in younger and older Chinese adults: a randomized, placebo‐controlled, double‐blind phase 1 study. Nat Med. 2021;27(6):1062‐1070. 10.1038/s41591-021-01330-9 [DOI] [PubMed] [Google Scholar]
- 47. Klein NP, Lewis N, Goddard K, et al. Surveillance for adverse events after COVID‐19 mRNA vaccination. JAMA. 2021;326:1390‐1399. 10.1001/jama.2021.15072 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Jeet Kaur R, Dutta S, Charan J, et al. Cardiovascular adverse events reported from COVID‐19 vaccines: a study based on WHO database. Int J Gen Med. 2021;14:3909‐3927. 10.2147/IJGM.S324349 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Gubernot D, Jazwa A, Niu M, et al. U.S. population‐based background incidence rates of medical conditions for use in safety assessment of COVID‐19 vaccines. Vaccine. 2021;39(28):3666‐3677. 10.1016/j.vaccine.2021.05.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Gargano JW, Wallace M, Hadler SC, et al. Use of mRNA COVID‐19 vaccine after reports of myocarditis among vaccine recipients: update from the advisory committee on immunization practices ‐ United States, June 2021. MMWR Morb Mortal Wkly Rep. 2021;70(27):977‐982. 10.15585/mmwr.mm7027e2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Mason JW, O'connell JB, Herskowitz A, et al. A clinical trial of immunosuppressive therapy for myocarditis. The myocarditis treatment trial investigators. N Engl J Med. 1995;333(5):269‐275. 10.1056/NEJM199508033330501 [DOI] [PubMed] [Google Scholar]
- 52. Grün S, Schumm J, Greulich S, et al. Long‐term follow‐up of biopsy‐proven viral myocarditis. J Am Coll Cardiol. 2012;59(18):1604‐1615. 10.1016/j.jacc.2012.01.007 [DOI] [PubMed] [Google Scholar]
- 53. Bardy GH, Lee KL, Mark DB, et al. Amiodarone or an implantable cardioverter‐defibrillator for congestive heart failure. N Engl J Med. 2005;352(3):225‐237. 10.1056/NEJMoa043399 [DOI] [PubMed] [Google Scholar]
- 54. Sundaram V, Fang JC. Gastrointestinal and liver issues in heart failure. Circulation. 2016;133(17):1696‐1703. 10.1161/CIRCULATIONAHA.115.020894 [DOI] [PubMed] [Google Scholar]
- 55. Krack A, Sharma R, Figulla HR, Anker SD. The importance of the gastrointestinal system in the pathogenesis of heart failure. Eur Heart J. 2005;26(22):2368‐2374. 10.1093/eurheartj/ehi389 [DOI] [PubMed] [Google Scholar]
- 56. Gelman S. Venous function and central venous pressure: a physiologic story. Anesthesiology. 2008;108(4):735‐748. 10.1097/ALN.0b013e3181672607 [DOI] [PubMed] [Google Scholar]
- 57. Brandtzaeg P, Halstensen TS, Kett K, et al. Immunobiology and immunopathology of human gut mucosa: humoral immunity and intraepithelial lymphocytes. Gastroenterology. 1989;97(6):1562‐1584. 10.1016/0016-5085(89)90406-x [DOI] [PubMed] [Google Scholar]
- 58. Rauchhaus M, Coats AJ, Anker SD. The endotoxin‐lipoprotein hypothesis. The Lancet. 2000;356(9233):930‐933. 10.1016/S0140-6736(00)02690-8 [DOI] [PubMed] [Google Scholar]
- 59. Deswal A, Petersen NJ, Feldman AM, Young JB, White BG, Mann DL. Cytokines and cytokine receptors in advanced heart failure. Circulation. 2001;103(16):2055‐2059. 10.1161/01.CIR.103.16.2055 [DOI] [PubMed] [Google Scholar]
- 60. Hsiao HJ, Hsia SH, Wu CT, et al. Clinical presentation of pediatric myocarditis in Taiwan. Pediatr Neonatol. 2011;52(3):135‐139. 10.1016/j.pedneo.2011.03.005 [DOI] [PubMed] [Google Scholar]
- 61. Chang YJ, Hsiao HJ, Hsia SH, et al. Analysis of clinical parameters and echocardiography as predictors of fatal pediatric myocarditis. PLoS One. 2019;14(3):e0214087. 10.1371/journal.pone.0214087 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Butts RJ, Boyle GJ, Deshpande SR, et al. Characteristics of clinically diagnosed pediatric myocarditis in a contemporary multi‐center cohort. Pediatr Cardiol. 2017;38(6):1175‐1182. 10.1007/s00246-017-1638-1 [DOI] [PubMed] [Google Scholar]
- 63. Caforio AL, Pankuweit S, Arbustini E, et al. Current state of knowledge on aetiology, diagnosis, management, and therapy of myocarditis: a position statement of the European Society of Cardiology Working Group on Myocardial and Pericardial Diseases. Eur Heart J. 2013;34(33):2636‐2648. 10.1093/eurheartj/eht210 [DOI] [PubMed] [Google Scholar]
- 64. Krammer F, Srivastava K, Alshammary H, et al. Antibody responses in seropositive persons after a single dose of SARS‐CoV‐2 mRNA vaccine. N Engl J Med. 2021;384(14):1372‐1374. 10.1056/NEJMc2101667 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Gaebler C, Wang Z, Lorenzi JCC, et al. Evolution of antibody immunity to SARS‐CoV‐2. Nature. 2021;591(7851):639‐644. 10.1038/s41586-021-03207-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Hékimian G, Kerneis M, Zeitouni M, et al. Coronavirus disease 2019 acute myocarditis and multisystem inflammatory syndrome in adult intensive and cardiac care units. Chest. 2021;159(2):657‐662. 10.1016/j.chest.2020.08.2099 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Abrams JY, Oster ME, Godfred‐Cato SE, et al. Factors linked to severe outcomes in multisystem inflammatory syndrome in children (MIS‐C) in the USA: a retrospective surveillance study. Lancet Child Adolesc Health. 2021;5(5):323‐331. 10.1016/S2352-4642(21)00050-X [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Siripanthong B, Nazarian S, Muser D, et al. Recognizing COVID‐19‐related myocarditis: the possible pathophysiology and proposed guideline for diagnosis and management. Heart Rhythm. 2020;17(9):1463‐1471. 10.1016/j.hrthm.2020.05.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Boehmer TK, Kompaniyets L, Lavery AM, et al. Association between COVID‐19 and myocarditis using hospital‐based administrative data ‐ United States, March 2020‐January 2021. MMWR Morb Mortal Wkly Rep. 2021;70(35):1228‐1232. 10.15585/mmwr.mm7035e5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Starekova J, Bluemke DA, Bradham WS, et al. Evaluation for myocarditis in competitive student athletes recovering from coronavirus disease 2019 with cardiac magnetic resonance imaging. JAMA Cardiol. 2021;6(8):945‐950. 10.1001/jamacardio.2020.7444 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Barda N, Dagan N, Ben‐Shlomo Y, et al. Safety of the BNT162b2 mRNA Covid‐19 vaccine in a nationwide setting. N Engl J Med. 2021;0(0), null 10.1056/NEJMoa2110475 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supporting information.
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.


