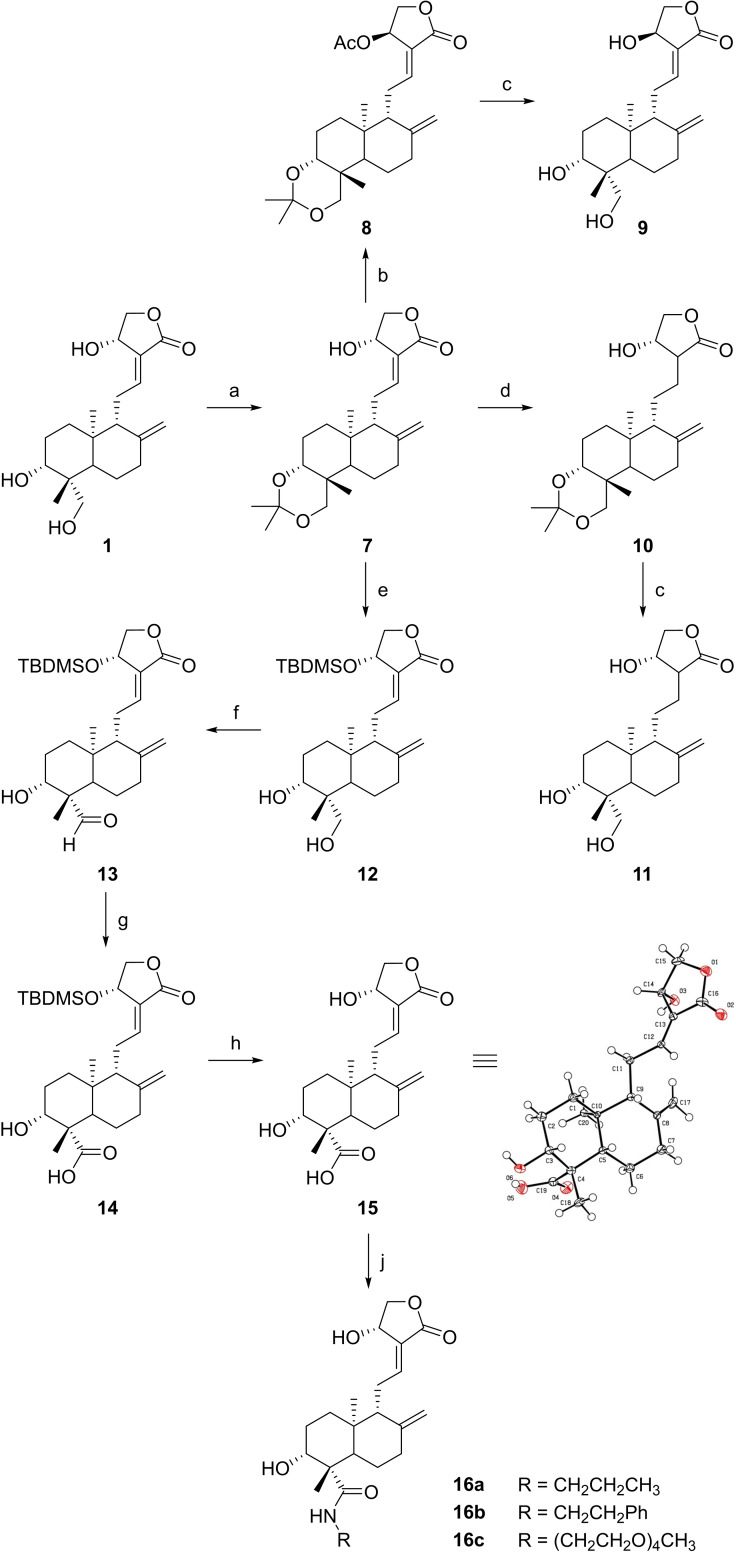
Scheme 2.
Synthesis of andrographolide derivatives. Reagents and conditions: (a) 2,2‐Dimethoxypropane, p‐TsOH, toluene, DMSO, rt, 6 h; (b) PPh3, DIAD, AcOH, THF, rt, 18 h; (c) p‐TsOH, MeOH, H2O, 40 °C, 4 h; (d) NiCl2, NaBH4, MeOH, 0 °C, 10 min; (e) (i) TBDMSCl, imidazole, DMF, rt, 18 h; (ii) AcOH, H2O, rt, 2 h; (f) TEMPO, TBAI, NCS, CH2Cl2, K2CO3/NaHCO3 buffer pH 8.6, rt, 18 h; (g) 2‐methyl‐2‐butene, NaClO2, NaH2PO4, tBuOH, THF, H2O, rt, 18 h; (h) AcCl, MeOH, rt, 1 h; (j) R‐NH2, HATU, DIPEA, DMF, rt, 18 h.