Extended Data Fig. 5 |. Unstimulated reversible FUS-CAR T in vivo control experiment associated with Fig. 6.
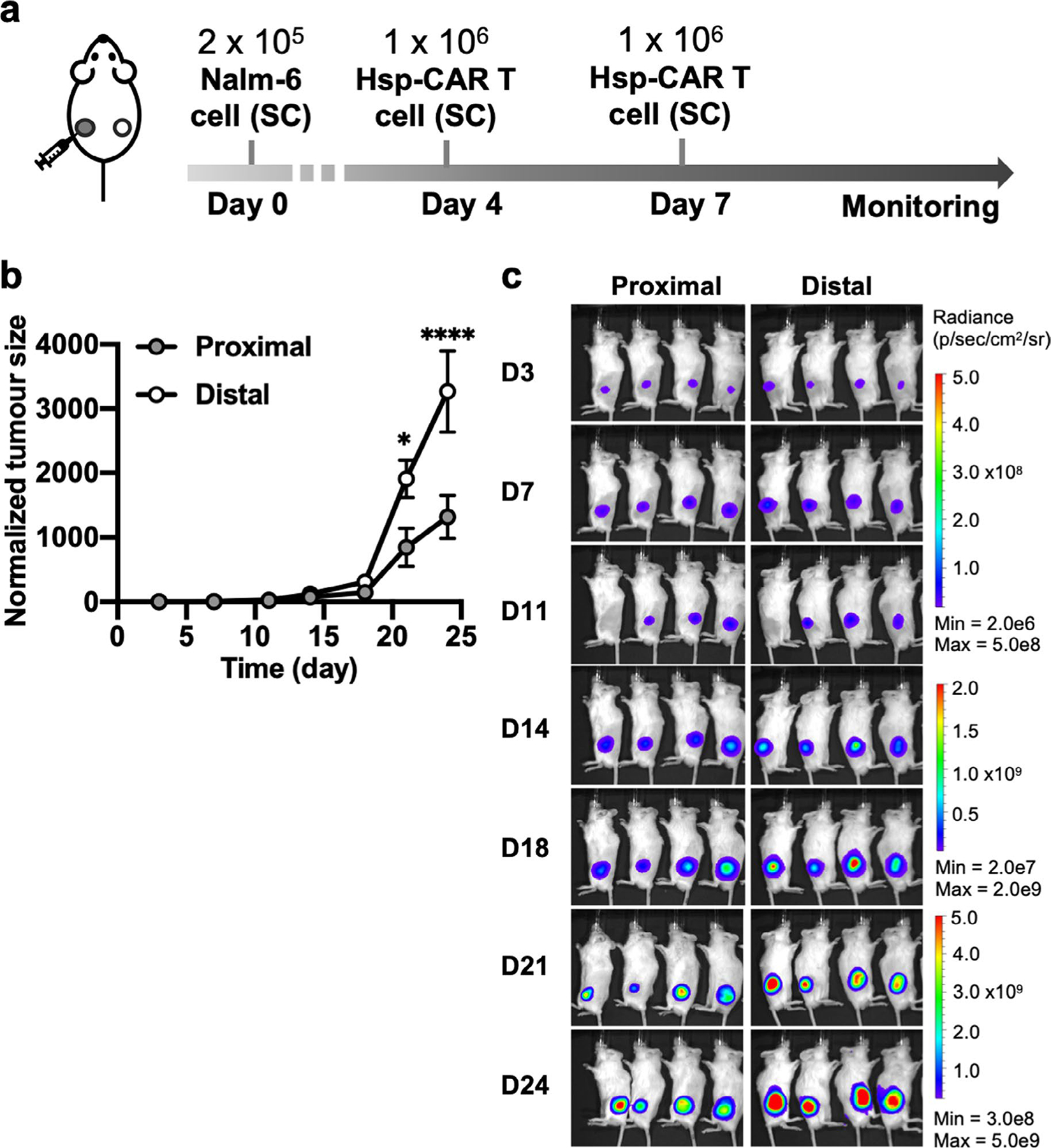
a, Timeline of the experiment. Bilateral tumour-bearing mice received local injections of the reversible FUS-CAR (Hsp-CAR) T cells without FUS stimulation at the left tumour (proximal) on Day 4 and Day 7. The right tumour (distal) received no treatment. b,c, Normalized tumour size (*P = 0.011, ****P = 1.4 × 10−5, Two-way ANOVA followed by Sidak’s multiple comparisons test) (b) and BLI images of the proximal and distal tumours (c). Tumour size was quantified using the integrated Fluc luminescence intensity of the tumour region and normalized to that of the same tumour on the first measurement. Data points and error bars represent means of 4 mice ± SEM.
