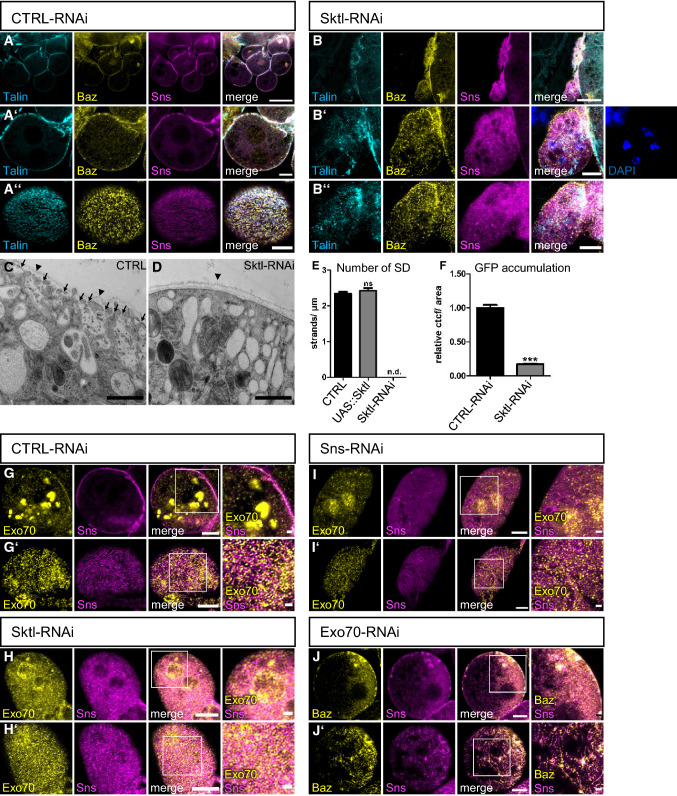
Fig. 2.
PI(4,5)P2 produced by Skittles is essential for slit diaphragm formation and endocytosis. (A, B) Garland nephrocytes from 3rd instar larvae expressing either control RNAi (A) or Sktl-RNAi (B) were stained with the indicated antibodies. (C, D) Transmission electron microscopy of garland nephrocytes of control third instar larvae (C) and Sktl-RNAi-expressing larvae (D). Some slit diaphragms were labeled with arrows in control nephrocytes. Slit diaphragms were absent in Sktl-RNAi expressing nephrocytes. Arrow heads mark the basement membrane. E Slit diaphragms of nephrocytes expressing Sktl or control RNAi were quantified from surface views. For this, a 5 µm line perpendicular to the Sns-strands was drawn and the number of strands quantified. 5 lines/nephrocyte and at least 5 nephrocytes were quantified per genotype. Sktl-RNAi expressing nephrocytes were not characterized as they did not display detectable Sns strands at the surface but exhibited a rather diffuse Sns staining. Significance was determined by Mann–Whitney test: n.s. not significant. n.d. not determined. F Endocytosis of a secreted ANP-2xGFP by garland nephrocytes expressing the indicated RNAi’s was quantified as described in the methods section. At least 100 nephrocytes from at least 15 different larvae were evaluated. Significance was determined by Mann–Whitney test: ***p < 0.001. (G-I) Nephrocytes expressing control RNAi (G), Sktl RNAi (H) and Sns RNAi (I) were co-stained with Exo70 and Sns. (J) Immunostainings of nephrocytes expressing Exo70-RNAi. Scales bars are 20 µm in A and B, 5 µm in A’, A’’, B’, B’’ and G–J, 1 µm in C, D and in insets in G’-J’. Error bars are standard error of the means