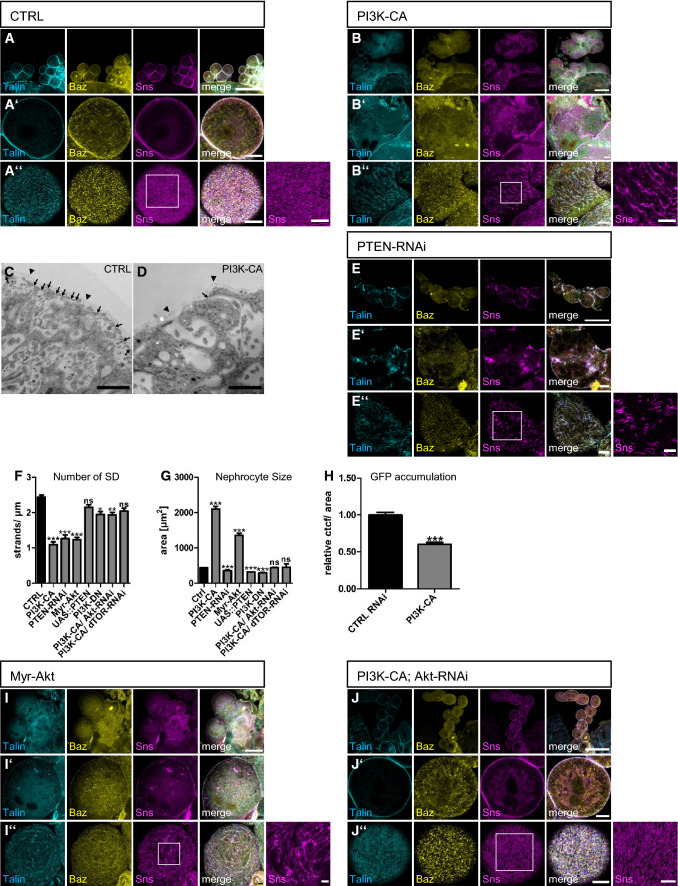
Fig. 3.
PI(3,4,5)P3 is not essential for slit diaphragm assembly and maintenance. (A, B) Garland nephrocytes from 3rd instar larvae either of controls (sns::GAL4 crossed with the empty attP40 line, (A) or of animals expressing a constitutively activated Pi3K (Pi3K-CA, B) in nephrocytes were stained with the indicated antibodies. C, D Transmission electron microscopy of garland nephrocytes of control third instar larvae (C) and PI3K-CA expressing larvae (D). Slit diaphragms were labeled with arrows and arrow heads mark the basement membrane. E Immunostainings of nephrocytes expressing RNAi against PTEN. F Slit diaphragms of nephrocytes expressing the indicated transgenes were quantified from surface views. For this, a 5 µm line perpendicular to the Sns-strands was drawn and the number of strands quantified. 5 lines/nephrocyte and at least 5 nephrocytes were quantified per genotype. Significance was determined by Kruskal–Wallis test and Dunn’s correction: ***p < 0.001, **p < 0.01, *p < 0.05, n.s. not significant. G The size of nephrocytes expressing the indicated transgenes was quantified by measuring the cell area of equatorial sections. At least 120 nephrocytes from at least 10 larvae were quantified. Significance was determined by Kruskal–Wallis test and Dunns correction: ***p < 0.001, n.s. not significant. H Endocytosis of a secreted ANP-2xGFP by garland nephrocytes expressing the indicated controls was quantified as described in the methods section. At least 100 nephrocytes from at least 15 different larvae were evaluated. Significance was determined by Mann–Whitney test: ***p < 0.001. I Myr-Akt expressing nephrocytes were stained with the indicated antibodies. J Immunostainings of nephrocytes expressing PI3K-CA together with RNAi targeting Akt. Scale bars are 50 µm in A, B, E, I and J, 5 µm in A’, A’’, B’, B’’, E’, E’’, I’, I’’, J’ and J’’, 2.5 µm in insets in A’’, B’’, E’’, I’’, J’’ and 1 µm in C and D. Error bars are standard error of the means