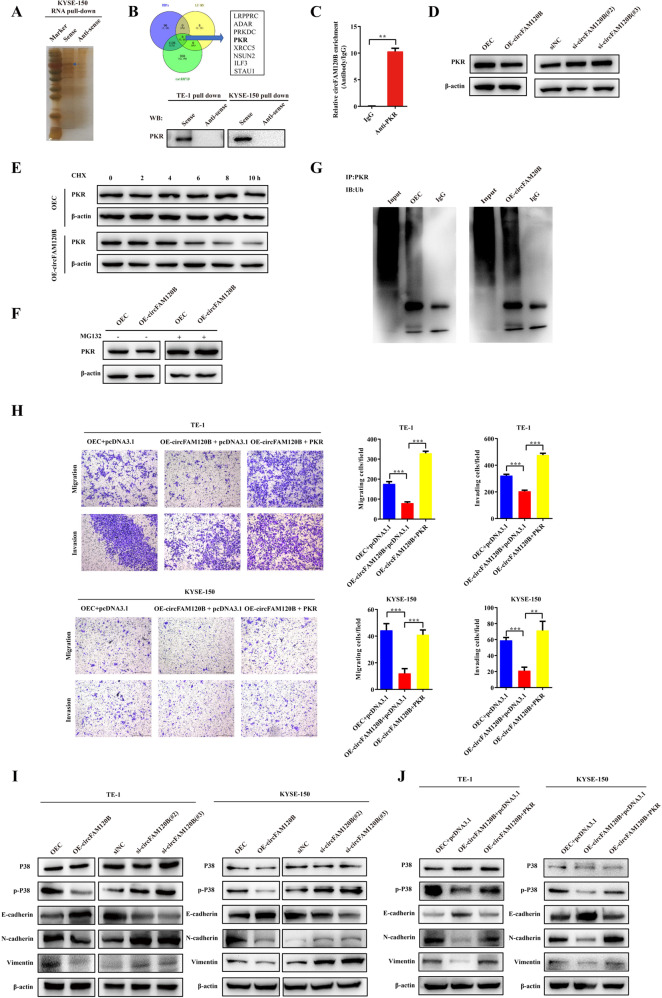
Fig. 5. circFAM120B physically interacts with PKR and promotes its ubiquitin/proteasome-mediated degradation.
A Silver staining of proteins pulled down by circFAM120B. B A total of 8 candidate proteins were identified after the intersection of the RNA pull-down dataset (peptides >5), website predictions (catRAPID, http://service.tartaglialab.com/page/catrapid_omics2_group), and classic RNA binding protein datasets. The specific amino acid sequences were detected by mass spectrometry. PKR was pulled down by a circFAM120B sense RNA probe but not by the antisense probe. C RIP assays with qRT-PCR show that circFAM120B was pulled down by an anti-PKR antibody in KYSE-150 cells. D The specific association of PKR and circFAM120B was detected by western blot analysis. E KYSE-150 cells stably overexpressing circFAM120B or controls were treated with cycloheximide (CHX, 50 µg/ml) at indicated time points and analyzed by western blot. F KYSE-150 cells stably overexpressing circFAM120B or controls were treated with MG132 (25 mmol/L) for 10 h and analyzed by western blot. β-actin was used as an internal control. G The ubiquitination of PKR was detected by western blot in KYSE-150 cells with or without circFAM120B overexpression. H Rescue experiments indicated that PKR was essential for circFAM120B-induced inhibition of migration and invasion. I, J Protein levels were evaluated by western blot assays in ESCC cells with the indicated treatments. **P < 0.01, ***P < 0.001.